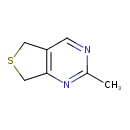| Record Information |
|---|
| Version | 1.0 |
|---|
| Creation Date | 2016-05-19 02:01:05 UTC |
|---|
| Update Date | 2016-11-09 01:09:31 UTC |
|---|
| Accession Number | CHEM005357 |
|---|
| Identification |
|---|
| Common Name | 5,7-DIHYDRO-2-METHYLTHIENO(3,4-D)PYRIMIDINE |
|---|
| Class | Small Molecule |
|---|
| Description | Cystine-derived Maillard produced 5,7-Dihydro-2-methylthieno[3,4-d]pyrimidine is a flavouring ingredient. Sweetness enhancer at low concentration (10 ppm), at higher concentration enhances root vegetable flavour in chicken broth base. |
|---|
| Contaminant Sources | - EAFUS Chemicals
- FooDB Chemicals
|
|---|
| Contaminant Type | Not Available |
|---|
| Chemical Structure | |
|---|
| Synonyms | | Value | Source |
|---|
| 2-Methyl-5,7-dihydrothieno(3,4-D)pyrimidine | HMDB | | 2-Methyl-5,7-dihydrothieno[3,4-D]pyrimidine | HMDB | | 5,7-dihydro-2-Methyl-thieno(3,4-D)pyrimidine | HMDB | | 5,7-dihydro-2-methylthieno(3,4-D)Pyrimidine | HMDB | | FEMA 3338 | HMDB |
|
|---|
| Chemical Formula | C7H8N2S |
|---|
| Average Molecular Mass | 152.220 g/mol |
|---|
| Monoisotopic Mass | 152.041 g/mol |
|---|
| CAS Registry Number | 36267-71-7 |
|---|
| IUPAC Name | 2-methyl-5H,7H-thieno[3,4-d]pyrimidine |
|---|
| Traditional Name | 2-methyl-5H,7H-thieno[3,4-d]pyrimidine |
|---|
| SMILES | Not Available |
|---|
| InChI Identifier | InChI=1S/C7H8N2S/c1-5-8-2-6-3-10-4-7(6)9-5/h2H,3-4H2,1H3 |
|---|
| InChI Key | XSUYIZJJKIKWFN-UHFFFAOYSA-N |
|---|
| Chemical Taxonomy |
|---|
| Description | belongs to the class of organic compounds known as thienopyrimidines. These are heterocyclic compounds containing a thiophene ring fused to a pyrimidine ring. Thiophene is 5-membered ring consisting of four carbon atoms and one sulfur atom. Pyrimidine is a 6-membered ring consisting of four carbon atoms and two nitrogen centers at the 1- and 3- ring positions. |
|---|
| Kingdom | Organic compounds |
|---|
| Super Class | Organoheterocyclic compounds |
|---|
| Class | Thienopyrimidines |
|---|
| Sub Class | Not Available |
|---|
| Direct Parent | Thienopyrimidines |
|---|
| Alternative Parents | |
|---|
| Substituents | - Thienopyrimidine
- Pyrimidine
- Heteroaromatic compound
- Azacycle
- Dialkylthioether
- Thioether
- Organic nitrogen compound
- Organopnictogen compound
- Hydrocarbon derivative
- Organonitrogen compound
- Aromatic heteropolycyclic compound
|
|---|
| Molecular Framework | Aromatic heteropolycyclic compounds |
|---|
| External Descriptors | Not Available |
|---|
| Biological Properties |
|---|
| Status | Detected and Not Quantified |
|---|
| Origin | Not Available |
|---|
| Cellular Locations | Not Available |
|---|
| Biofluid Locations | Not Available |
|---|
| Tissue Locations | Not Available |
|---|
| Pathways | Not Available |
|---|
| Applications | Not Available |
|---|
| Biological Roles | Not Available |
|---|
| Chemical Roles | Not Available |
|---|
| Physical Properties |
|---|
| State | Not Available |
|---|
| Appearance | Not Available |
|---|
| Experimental Properties | | Property | Value |
|---|
| Melting Point | Not Available | | Boiling Point | Not Available | | Solubility | Not Available |
|
|---|
| Predicted Properties | |
|---|
| Spectra |
|---|
| Spectra | | Spectrum Type | Description | Splash Key | View |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | splash10-0zfr-2900000000-2474de04133f8444b981 | Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | Not Available | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-0udi-0900000000-f6b8a53870c07c0a2e75 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-0udi-0900000000-9819704a57b38b49c4ee | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-08i9-6900000000-a063ca9f92d029e7ac00 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-0udi-0900000000-3504fcdb5dc65eb2ace3 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-0udi-1900000000-b278a607d9cf01e1b7c8 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-000i-4900000000-514cde59921747f51737 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-0udi-2900000000-57620acde88986802656 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-0udi-0900000000-5b56ad2ab5e55d64664d | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-014l-9300000000-2446811585bd4c107985 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-0udi-0900000000-eedac6a369762f424a1e | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-0zfr-0900000000-e06c79dfaf7b4a5ed992 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-052s-3900000000-f7fd8cb746ee60746af2 | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum |
|
|---|
| Toxicity Profile |
|---|
| Route of Exposure | Not Available |
|---|
| Mechanism of Toxicity | Not Available |
|---|
| Metabolism | Not Available |
|---|
| Toxicity Values | Not Available |
|---|
| Lethal Dose | Not Available |
|---|
| Carcinogenicity (IARC Classification) | Not Available |
|---|
| Uses/Sources | Not Available |
|---|
| Minimum Risk Level | Not Available |
|---|
| Health Effects | Not Available |
|---|
| Symptoms | Not Available |
|---|
| Treatment | Not Available |
|---|
| Concentrations |
|---|
| Not Available |
|---|
| External Links |
|---|
| DrugBank ID | Not Available |
|---|
| HMDB ID | HMDB0041565 |
|---|
| FooDB ID | FDB021555 |
|---|
| Phenol Explorer ID | Not Available |
|---|
| KNApSAcK ID | Not Available |
|---|
| BiGG ID | Not Available |
|---|
| BioCyc ID | Not Available |
|---|
| METLIN ID | Not Available |
|---|
| PDB ID | Not Available |
|---|
| Wikipedia Link | Not Available |
|---|
| Chemspider ID | 55809 |
|---|
| ChEBI ID | Not Available |
|---|
| PubChem Compound ID | 61951 |
|---|
| Kegg Compound ID | Not Available |
|---|
| YMDB ID | Not Available |
|---|
| ECMDB ID | Not Available |
|---|
| References |
|---|
| Synthesis Reference | Not Available |
|---|
| MSDS | Not Available |
|---|
| General References | |
|---|