| Record Information |
|---|
| Version | 1.0 |
|---|
| Creation Date | 2016-05-19 02:01:03 UTC |
|---|
| Update Date | 2016-11-09 01:09:31 UTC |
|---|
| Accession Number | CHEM005354 |
|---|
| Identification |
|---|
| Common Name | DIHYDRO-BETA-IONOL |
|---|
| Class | Small Molecule |
|---|
| Description | 4-(2,6,6-Trimethyl-1-cyclohexenyl)-2-butanol is found in fruits. 4-(2,6,6-Trimethyl-1-cyclohexenyl)-2-butanol is present in loganberry, Chinese quince oil, yellow passion fruit, Chinese scented green tea. 4-(2,6,6-Trimethyl-1-cyclohexenyl)-2-butanol is a flavouring ingredient |
|---|
| Contaminant Sources | - EAFUS Chemicals
- FooDB Chemicals
|
|---|
| Contaminant Type | Not Available |
|---|
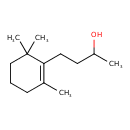
| Chemical Structure | |
|---|
| Synonyms | | Value | Source |
|---|
| 4-(2,6,6-Trimethyl-1-cyclohexen-1-yl)-2-butanol | HMDB | | 4-(2,6,6-Trimethyl-1-cyclohexenyl)butan-2-ol | HMDB | | 4-(2,6,6-Trimethyl-cyclohex-1-enyl)-butan-2-ol | HMDB | | 7,8-dihydro-b-Ionol | HMDB | | 7,8-dihydro-beta -Ionol | HMDB | | a,2,6,6-Tetramethyl-1-cyclohexene-1-propanol, 9ci | HMDB | | alpha,2,6,6-Tetramethyl-1-cyclohexene-1-propanol | HMDB | | alpha,2,6,6-Tetramethylcyclohexene-1-propan-1-ol | HMDB | | dihydro- beta -Ionol | HMDB | | dihydro-beta-Ionol | HMDB | | FEMA 3627 | HMDB |
|
|---|
| Chemical Formula | C13H24O |
|---|
| Average Molecular Mass | 196.329 g/mol |
|---|
| Monoisotopic Mass | 196.183 g/mol |
|---|
| CAS Registry Number | 3293-47-8 |
|---|
| IUPAC Name | 4-(2,6,6-trimethylcyclohex-1-en-1-yl)butan-2-ol |
|---|
| Traditional Name | 4-(2,6,6-trimethylcyclohex-1-en-1-yl)butan-2-ol |
|---|
| SMILES | CC(O)CCC1=C(C)CCCC1(C)C |
|---|
| InChI Identifier | InChI=1S/C13H24O/c1-10-6-5-9-13(3,4)12(10)8-7-11(2)14/h11,14H,5-9H2,1-4H3 |
|---|
| InChI Key | VSYLEWGIVLSDIY-UHFFFAOYSA-N |
|---|
| Chemical Taxonomy |
|---|
| Description | belongs to the class of organic compounds known as sesquiterpenoids. These are terpenes with three consecutive isoprene units. |
|---|
| Kingdom | Organic compounds |
|---|
| Super Class | Lipids and lipid-like molecules |
|---|
| Class | Prenol lipids |
|---|
| Sub Class | Sesquiterpenoids |
|---|
| Direct Parent | Sesquiterpenoids |
|---|
| Alternative Parents | |
|---|
| Substituents | - Megastigmane sesquiterpenoid
- Sesquiterpenoid
- Secondary alcohol
- Organic oxygen compound
- Hydrocarbon derivative
- Organooxygen compound
- Alcohol
- Aliphatic homomonocyclic compound
|
|---|
| Molecular Framework | Aliphatic homomonocyclic compounds |
|---|
| External Descriptors | Not Available |
|---|
| Biological Properties |
|---|
| Status | Detected and Not Quantified |
|---|
| Origin | Not Available |
|---|
| Cellular Locations | Not Available |
|---|
| Biofluid Locations | Not Available |
|---|
| Tissue Locations | Not Available |
|---|
| Pathways | Not Available |
|---|
| Applications | Not Available |
|---|
| Biological Roles | Not Available |
|---|
| Chemical Roles | Not Available |
|---|
| Physical Properties |
|---|
| State | Not Available |
|---|
| Appearance | Not Available |
|---|
| Experimental Properties | | Property | Value |
|---|
| Melting Point | Not Available | | Boiling Point | Not Available | | Solubility | Not Available |
|
|---|
| Predicted Properties | |
|---|
| Spectra |
|---|
| Spectra | | Spectrum Type | Description | Splash Key | View |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | splash10-0fas-5900000000-fabe419ab558385d270b | Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (1 TMS) - 70eV, Positive | splash10-0uki-9560000000-7aa09705e4bb20569062 | Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | Not Available | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-004i-1900000000-2597ce99e272cbc150ad | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-004i-4900000000-d6904b3d805591b4a436 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-0673-9700000000-69ad6cb4fc8d400a20c9 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-0002-0900000000-ff71df797386c8663f77 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-002b-0900000000-69c51fa80abb11f65c8a | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-0570-4900000000-069aae98916c2590810f | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-05bb-3900000000-2af1a7c112b8e31f8ec4 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-00dr-5900000000-a6751ed948a09e250802 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-066r-9300000000-249a92cbe6970448fcb8 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-0002-0900000000-e1aa8d629d4398db9400 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-0fdk-0900000000-d55c9fd1171b878abaa6 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-0002-1900000000-b6174cc4127879282d74 | Spectrum |
|
|---|
| Toxicity Profile |
|---|
| Route of Exposure | Not Available |
|---|
| Mechanism of Toxicity | Not Available |
|---|
| Metabolism | Not Available |
|---|
| Toxicity Values | Not Available |
|---|
| Lethal Dose | Not Available |
|---|
| Carcinogenicity (IARC Classification) | Not Available |
|---|
| Uses/Sources | Not Available |
|---|
| Minimum Risk Level | Not Available |
|---|
| Health Effects | Not Available |
|---|
| Symptoms | Not Available |
|---|
| Treatment | Not Available |
|---|
| Concentrations |
|---|
| Not Available |
|---|
| External Links |
|---|
| DrugBank ID | Not Available |
|---|
| HMDB ID | HMDB0036172 |
|---|
| FooDB ID | FDB015026 |
|---|
| Phenol Explorer ID | Not Available |
|---|
| KNApSAcK ID | Not Available |
|---|
| BiGG ID | Not Available |
|---|
| BioCyc ID | Not Available |
|---|
| METLIN ID | Not Available |
|---|
| PDB ID | Not Available |
|---|
| Wikipedia Link | Not Available |
|---|
| Chemspider ID | 503562 |
|---|
| ChEBI ID | Not Available |
|---|
| PubChem Compound ID | 579336 |
|---|
| Kegg Compound ID | Not Available |
|---|
| YMDB ID | Not Available |
|---|
| ECMDB ID | Not Available |
|---|
| References |
|---|
| Synthesis Reference | Not Available |
|---|
| MSDS | Not Available |
|---|
| General References | |
|---|