| Record Information |
|---|
| Version | 1.0 |
|---|
| Creation Date | 2016-05-19 02:00:40 UTC |
|---|
| Update Date | 2016-11-09 01:09:31 UTC |
|---|
| Accession Number | CHEM005327 |
|---|
| Identification |
|---|
| Common Name | DIETHYL MALATE |
|---|
| Class | Small Molecule |
|---|
| Description | A malate ester obtained by the formal condensation of the two carboxy groups of malic acid with two molecules of ethanol respectively. |
|---|
| Contaminant Sources | - EAFUS Chemicals
- FooDB Chemicals
|
|---|
| Contaminant Type | Not Available |
|---|
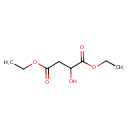
| Chemical Structure | |
|---|
| Synonyms | | Value | Source |
|---|
| Diethyl L-malic acid | Generator | | Butanedioic acid, 2-hydroxy-, 1,4-diethyl ester | HMDB | | Butanedioic acid, hydroxy-, diethyl ester | HMDB | | Diethyl (1)-malate | HMDB | | Diethyl hydroxybutanedioate | HMDB | | Diethyl hydroxybutanoate | HMDB | | Diethyl malate | HMDB | | Ethyl DL-malate | HMDB | | Ethyl malate | HMDB | | Ethyl-DL-malate | HMDB | | Hydroxy-diethyl ester(.+/-.)-butanedioic acid | HMDB | | L-(-)-Malic acid diethyl ester | HMDB | | Malic acid, diethyl ester | HMDB | | Diethyl malate, (R)-isomer | MeSH, HMDB | | Diethyl malate, (+-)-isomer | MeSH, HMDB | | Diethyl malate, (S)-isomer | MeSH, HMDB | | Diethyl malic acid | Generator | | Diethyl D-malic acid | Generator |
|
|---|
| Chemical Formula | C8H14O5 |
|---|
| Average Molecular Mass | 190.194 g/mol |
|---|
| Monoisotopic Mass | 190.084 g/mol |
|---|
| CAS Registry Number | 7554-12-3 |
|---|
| IUPAC Name | 1,4-diethyl 2-hydroxybutanedioate |
|---|
| Traditional Name | 1,4-diethyl 2-hydroxybutanedioate |
|---|
| SMILES | CCOC(=O)CC(O)C(=O)OCC |
|---|
| InChI Identifier | InChI=1S/C8H14O5/c1-3-12-7(10)5-6(9)8(11)13-4-2/h6,9H,3-5H2,1-2H3 |
|---|
| InChI Key | VKNUORWMCINMRB-UHFFFAOYSA-N |
|---|
| Chemical Taxonomy |
|---|
| Description | belongs to the class of organic compounds known as beta hydroxy acids and derivatives. Beta hydroxy acids and derivatives are compounds containing a carboxylic acid substituted with a hydroxyl group on the C3 carbon atom. |
|---|
| Kingdom | Organic compounds |
|---|
| Super Class | Organic acids and derivatives |
|---|
| Class | Hydroxy acids and derivatives |
|---|
| Sub Class | Beta hydroxy acids and derivatives |
|---|
| Direct Parent | Beta hydroxy acids and derivatives |
|---|
| Alternative Parents | |
|---|
| Substituents | - Fatty acid ester
- Beta-hydroxy acid
- Fatty acyl
- Dicarboxylic acid or derivatives
- Secondary alcohol
- Carboxylic acid ester
- Carboxylic acid derivative
- Organic oxygen compound
- Organic oxide
- Hydrocarbon derivative
- Organooxygen compound
- Carbonyl group
- Alcohol
- Aliphatic acyclic compound
|
|---|
| Molecular Framework | Aliphatic acyclic compounds |
|---|
| External Descriptors | |
|---|
| Biological Properties |
|---|
| Status | Detected and Not Quantified |
|---|
| Origin | Not Available |
|---|
| Cellular Locations | Not Available |
|---|
| Biofluid Locations | Not Available |
|---|
| Tissue Locations | Not Available |
|---|
| Pathways | Not Available |
|---|
| Applications | Not Available |
|---|
| Biological Roles | Not Available |
|---|
| Chemical Roles | Not Available |
|---|
| Physical Properties |
|---|
| State | Not Available |
|---|
| Appearance | Not Available |
|---|
| Experimental Properties | | Property | Value |
|---|
| Melting Point | Not Available | | Boiling Point | Not Available | | Solubility | Not Available |
|
|---|
| Predicted Properties | |
|---|
| Spectra |
|---|
| Spectra | | Spectrum Type | Description | Splash Key | View |
|---|
| GC-MS | GC-MS Spectrum - EI-B (Non-derivatized) | splash10-00vl-9200000000-0e2b63fa1358ecc4727d | Spectrum | | GC-MS | GC-MS Spectrum - EI-B (Non-derivatized) | splash10-00vl-9200000000-0e2b63fa1358ecc4727d | Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | splash10-014i-9800000000-c3cb88ecf650c67efe5e | Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (1 TMS) - 70eV, Positive | splash10-0079-8920000000-732bc15836f011a383a3 | Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | Not Available | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-0007-1900000000-815e69499173edb4db80 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-000b-9800000000-b787dba980fd1db16e61 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-0002-9200000000-4e46a07b9fd73378c9ca | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-000l-3900000000-f255ae39fead1d6d56dd | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-0075-9800000000-916e9fe90e1aecbe634d | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-00dv-9100000000-c36e5fae8fc58b06cf20 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-0079-3900000000-349bf26b0d73cd973a8c | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-00fv-9400000000-2464ee9878c2e2c698a0 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-0076-9000000000-888ebde00273a66ebeb0 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-00kb-3900000000-8c2cbe244fa84072171c | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-0fxt-8900000000-7b232d5d7b06aaf439bb | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-006w-9100000000-a25e4bfa8d5a0b9203da | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum |
|
|---|
| Toxicity Profile |
|---|
| Route of Exposure | Not Available |
|---|
| Mechanism of Toxicity | Not Available |
|---|
| Metabolism | Not Available |
|---|
| Toxicity Values | Not Available |
|---|
| Lethal Dose | Not Available |
|---|
| Carcinogenicity (IARC Classification) | Not Available |
|---|
| Uses/Sources | Not Available |
|---|
| Minimum Risk Level | Not Available |
|---|
| Health Effects | Not Available |
|---|
| Symptoms | Not Available |
|---|
| Treatment | Not Available |
|---|
| Concentrations |
|---|
| Not Available |
|---|
| External Links |
|---|
| DrugBank ID | Not Available |
|---|
| HMDB ID | HMDB0040220 |
|---|
| FooDB ID | FDB019933 |
|---|
| Phenol Explorer ID | Not Available |
|---|
| KNApSAcK ID | Not Available |
|---|
| BiGG ID | Not Available |
|---|
| BioCyc ID | Not Available |
|---|
| METLIN ID | Not Available |
|---|
| PDB ID | Not Available |
|---|
| Wikipedia Link | Not Available |
|---|
| Chemspider ID | 22619 |
|---|
| ChEBI ID | 87368 |
|---|
| PubChem Compound ID | 24197 |
|---|
| Kegg Compound ID | Not Available |
|---|
| YMDB ID | YMDB01408 |
|---|
| ECMDB ID | Not Available |
|---|
| References |
|---|
| Synthesis Reference | Not Available |
|---|
| MSDS | Not Available |
|---|
| General References | |
|---|