| Record Information |
|---|
| Version | 1.0 |
|---|
| Creation Date | 2016-05-19 02:00:16 UTC |
|---|
| Update Date | 2016-11-09 01:09:31 UTC |
|---|
| Accession Number | CHEM005294 |
|---|
| Identification |
|---|
| Common Name | 7-DECEN-4-OLIDE |
|---|
| Class | Small Molecule |
|---|
| Description | (R)-5,6-Dihydro-6-pentyl-2H-pyran-2-one is used as a food additive [EAFUS] ("EAFUS: Everything Added to Food in the United States. [http://www.eafus.com/]") |
|---|
| Contaminant Sources | - EAFUS Chemicals
- FooDB Chemicals
|
|---|
| Contaminant Type | Not Available |
|---|
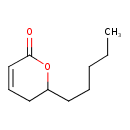
| Chemical Structure | |
|---|
| Synonyms | | Value | Source |
|---|
| (R)-5,6-dihydro-6-Pentyl-2H-pyran-2-one | HMDB | | 2-Decen-5-olide | HMDB | | 5,6-dihydro-6-Pentyl-(6R)-2H-pyran-2-one | HMDB | | 5,6-dihydro-6-Pentyl-(R)-2H-pyran-2-one | HMDB | | 5,6-dihydro-6-Pentyl-(theta)-2H-pyran-2-one | HMDB | | 5-Hydroxy-2-decenoic acid D-lactone | HMDB | | 5-Hydroxy-2-decenoic acid delta-lactone | HMDB | | 5-Hydroxy-2-decenoic acid lactone | HMDB | | 5-Hydroxy-2-decenoic acid laquo deltaraquo -lactone | HMDB | | 5-Hydroxy-2-decenoic acid, delta-lactone | HMDB | | 5-Hydroxy-2-decenoic acid, lactone | HMDB | | C-10 Massoia lactone | HMDB | | Cocolactone | HMDB | | FEMA 3744 | HMDB | | Massoia lactone | HMDB | | Massoilactone | HMDB | | Massoy lactone | HMDB |
|
|---|
| Chemical Formula | C10H16O2 |
|---|
| Average Molecular Mass | 168.233 g/mol |
|---|
| Monoisotopic Mass | 168.115 g/mol |
|---|
| CAS Registry Number | 67114-38-9 |
|---|
| IUPAC Name | 6-pentyl-5,6-dihydro-2H-pyran-2-one |
|---|
| Traditional Name | massoia lactone |
|---|
| SMILES | CCCCCC1CC=CC(=O)O1 |
|---|
| InChI Identifier | InChI=1S/C10H16O2/c1-2-3-4-6-9-7-5-8-10(11)12-9/h5,8-9H,2-4,6-7H2,1H3 |
|---|
| InChI Key | NEDIAPMWNCQWNW-UHFFFAOYSA-N |
|---|
| Chemical Taxonomy |
|---|
| Description | belongs to the class of organic compounds known as dihydropyranones. Dihydropyranones are compounds containing a hydrogenated pyran ring which bears a ketone, and contains one double bond. |
|---|
| Kingdom | Organic compounds |
|---|
| Super Class | Organoheterocyclic compounds |
|---|
| Class | Pyrans |
|---|
| Sub Class | Pyranones and derivatives |
|---|
| Direct Parent | Dihydropyranones |
|---|
| Alternative Parents | |
|---|
| Substituents | - Dihydropyranone
- Alpha,beta-unsaturated carboxylic ester
- Enoate ester
- Lactone
- Carboxylic acid ester
- Oxacycle
- Monocarboxylic acid or derivatives
- Carboxylic acid derivative
- Organic oxygen compound
- Organic oxide
- Hydrocarbon derivative
- Organooxygen compound
- Carbonyl group
- Aliphatic heteromonocyclic compound
|
|---|
| Molecular Framework | Aliphatic heteromonocyclic compounds |
|---|
| External Descriptors | Not Available |
|---|
| Biological Properties |
|---|
| Status | Detected and Not Quantified |
|---|
| Origin | Not Available |
|---|
| Cellular Locations | Not Available |
|---|
| Biofluid Locations | Not Available |
|---|
| Tissue Locations | Not Available |
|---|
| Pathways | Not Available |
|---|
| Applications | Not Available |
|---|
| Biological Roles | Not Available |
|---|
| Chemical Roles | Not Available |
|---|
| Physical Properties |
|---|
| State | Not Available |
|---|
| Appearance | Not Available |
|---|
| Experimental Properties | | Property | Value |
|---|
| Melting Point | Not Available | | Boiling Point | Not Available | | Solubility | Not Available |
|
|---|
| Predicted Properties | |
|---|
| Spectra |
|---|
| Spectra | | Spectrum Type | Description | Splash Key | View |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | splash10-0a6u-9200000000-4838fa181a658e937981 | Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | Not Available | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-066r-1900000000-38a27e29eba97dd46ed4 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-053r-9400000000-079af338b6c63fdba27f | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-0k96-9000000000-6bdc9ad8f20b1cc6ee7d | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-01b9-0900000000-1cddb2d1bcb26b9ce888 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-01b9-4900000000-5d0c17343ae1fcb5b046 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-006x-9200000000-06a261e45e734d2f0c9b | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-014i-0900000000-cb7592114e600c180aec | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-014i-1900000000-7ef5ea65442ecdc51768 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-066u-9100000000-9f4927d55fba82456126 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-00r6-9300000000-0b39ac8f80afa89ab89b | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-052f-9200000000-0d14ea24818166e85bf0 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-05mo-9000000000-e4d8d4609a670f215d4d | Spectrum |
|
|---|
| Toxicity Profile |
|---|
| Route of Exposure | Not Available |
|---|
| Mechanism of Toxicity | Not Available |
|---|
| Metabolism | Not Available |
|---|
| Toxicity Values | Not Available |
|---|
| Lethal Dose | Not Available |
|---|
| Carcinogenicity (IARC Classification) | Not Available |
|---|
| Uses/Sources | Not Available |
|---|
| Minimum Risk Level | Not Available |
|---|
| Health Effects | Not Available |
|---|
| Symptoms | Not Available |
|---|
| Treatment | Not Available |
|---|
| Concentrations |
|---|
| Not Available |
|---|
| External Links |
|---|
| DrugBank ID | Not Available |
|---|
| HMDB ID | HMDB0034452 |
|---|
| FooDB ID | FDB012860 |
|---|
| Phenol Explorer ID | Not Available |
|---|
| KNApSAcK ID | Not Available |
|---|
| BiGG ID | Not Available |
|---|
| BioCyc ID | Not Available |
|---|
| METLIN ID | Not Available |
|---|
| PDB ID | Not Available |
|---|
| Wikipedia Link | Not Available |
|---|
| Chemspider ID | 36500 |
|---|
| ChEBI ID | Not Available |
|---|
| PubChem Compound ID | 39914 |
|---|
| Kegg Compound ID | Not Available |
|---|
| YMDB ID | Not Available |
|---|
| ECMDB ID | Not Available |
|---|
| References |
|---|
| Synthesis Reference | Not Available |
|---|
| MSDS | Not Available |
|---|
| General References | |
|---|