| Record Information |
|---|
| Version | 1.0 |
|---|
| Creation Date | 2016-05-19 01:59:07 UTC |
|---|
| Update Date | 2016-11-09 01:09:30 UTC |
|---|
| Accession Number | CHEM005196 |
|---|
| Identification |
|---|
| Common Name | COCOA WITH DIOCTYL SODIUM SULFOSUCCINATE |
|---|
| Class | Small Molecule |
|---|
| Description | The sodium salt of diclofenac. |
|---|
| Contaminant Sources | - EAFUS Chemicals
- HPV EPA Chemicals
- IARC Carcinogens Group 2B
- OECD HPV Chemicals
- STOFF IDENT Compounds
- ToxCast & Tox21 Chemicals
|
|---|
| Contaminant Type | Not Available |
|---|
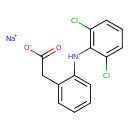
| Chemical Structure | |
|---|
| Synonyms | | Value | Source |
|---|
| (O-(2,6-Dichloroanilino)phenyl)acetic acid monosodium salt | ChEBI | | (O-(2,6-Dichloroanilino)phenyl)acetic acid sodium salt | ChEBI | | 2-((2,6-Dichlorophenyl)amino)benzeneacetic acid monosodium salt | ChEBI | | Sodium (O-((2,6-dichlorophenyl)amino)phenyl)acetate | ChEBI | | Sodium (O-(2,6-dichloroanilino)phenyl)acetate | ChEBI | | Sodium diclofenac | ChEBI | | Solaraze | Kegg | | Voltaren | Kegg | | (O-(2,6-Dichloroanilino)phenyl)acetate monosodium salt | Generator | | (O-(2,6-Dichloroanilino)phenyl)acetate sodium salt | Generator | | 2-((2,6-Dichlorophenyl)amino)benzeneacetate monosodium salt | Generator | | Sodium (O-((2,6-dichlorophenyl)amino)phenyl)acetic acid | Generator | | Sodium (O-(2,6-dichloroanilino)phenyl)acetic acid | Generator | | Diclofenac potassium | MeSH | | Diclonate P | MeSH | | Dicrofenac | MeSH | | Novapirina | MeSH | | Orthofen | MeSH | | Orthophen | MeSH | | Diclofenac, sodium | MeSH | | Feloran | MeSH | | SR 38 | MeSH | | SR-38 | MeSH | | Dichlofenal | MeSH | | Diclofenac | MeSH | | Diclophenac | MeSH | | Ortofen | MeSH | | Voltarol | MeSH |
|
|---|
| Chemical Formula | C14H10Cl2NNaO2 |
|---|
| Average Molecular Mass | 318.130 g/mol |
|---|
| Monoisotopic Mass | 316.999 g/mol |
|---|
| CAS Registry Number | 977038-67-7 |
|---|
| IUPAC Name | sodium 2-{2-[(2,6-dichlorophenyl)amino]phenyl}acetate |
|---|
| Traditional Name | sodium diclofenac(1-) |
|---|
| SMILES | [Na+].[O-]C(=O)CC1=CC=CC=C1NC1=C(Cl)C=CC=C1Cl |
|---|
| InChI Identifier | InChI=1S/C14H11Cl2NO2.Na/c15-10-5-3-6-11(16)14(10)17-12-7-2-1-4-9(12)8-13(18)19;/h1-7,17H,8H2,(H,18,19);/q;+1/p-1 |
|---|
| InChI Key | KPHWPUGNDIVLNH-UHFFFAOYSA-M |
|---|
| Chemical Taxonomy |
|---|
| Description | belongs to the class of organic compounds known as dichlorobenzenes. Dichlorobenzenes are compounds containing a benzene with exactly two chlorine atoms attached to it. |
|---|
| Kingdom | Organic compounds |
|---|
| Super Class | Benzenoids |
|---|
| Class | Benzene and substituted derivatives |
|---|
| Sub Class | Halobenzenes |
|---|
| Direct Parent | Dichlorobenzenes |
|---|
| Alternative Parents | |
|---|
| Substituents | - Aniline or substituted anilines
- 1,3-dichlorobenzene
- Aryl chloride
- Aryl halide
- Amino acid or derivatives
- Amino acid
- Carboxylic acid salt
- Organic metal halide
- Carboxylic acid derivative
- Organic alkali metal salt
- Carboxylic acid
- Monocarboxylic acid or derivatives
- Secondary amine
- Amine
- Organooxygen compound
- Organonitrogen compound
- Organochloride
- Organohalogen compound
- Organic zwitterion
- Organic salt
- Organic sodium salt
- Hydrocarbon derivative
- Organic oxide
- Carbonyl group
- Organopnictogen compound
- Organic oxygen compound
- Organic nitrogen compound
- Aromatic homomonocyclic compound
|
|---|
| Molecular Framework | Aromatic homomonocyclic compounds |
|---|
| External Descriptors | |
|---|
| Biological Properties |
|---|
| Status | Detected and Not Quantified |
|---|
| Origin | Not Available |
|---|
| Cellular Locations | Not Available |
|---|
| Biofluid Locations | Not Available |
|---|
| Tissue Locations | Not Available |
|---|
| Pathways | Not Available |
|---|
| Applications | Not Available |
|---|
| Biological Roles | Not Available |
|---|
| Chemical Roles | Not Available |
|---|
| Physical Properties |
|---|
| State | Not Available |
|---|
| Appearance | Not Available |
|---|
| Experimental Properties | | Property | Value |
|---|
| Melting Point | Not Available | | Boiling Point | Not Available | | Solubility | Not Available |
|
|---|
| Predicted Properties | |
|---|
| Spectra |
|---|
| Spectra | | Spectrum Type | Description | Splash Key | View |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-01dj-0093000000-ec15020bf39389b34080 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-0fk9-0090000000-95e0117ee87e73add8e1 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-0wvi-5490000000-95dea12c048c839ef488 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-014i-0039000000-c18bca80c357cb0f3f6f | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-014i-0098000000-da31f34e4a3afd6c3931 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-0002-0290000000-b6a1558b87939732cdd2 | Spectrum |
|
|---|
| Toxicity Profile |
|---|
| Route of Exposure | Not Available |
|---|
| Mechanism of Toxicity | Not Available |
|---|
| Metabolism | Not Available |
|---|
| Toxicity Values | Not Available |
|---|
| Lethal Dose | Not Available |
|---|
| Carcinogenicity (IARC Classification) | Not Available |
|---|
| Uses/Sources | Not Available |
|---|
| Minimum Risk Level | Not Available |
|---|
| Health Effects | Not Available |
|---|
| Symptoms | Not Available |
|---|
| Treatment | Not Available |
|---|
| Concentrations |
|---|
| Not Available |
|---|
| External Links |
|---|
| DrugBank ID | DBSALT000466 |
|---|
| HMDB ID | Not Available |
|---|
| FooDB ID | Not Available |
|---|
| Phenol Explorer ID | Not Available |
|---|
| KNApSAcK ID | Not Available |
|---|
| BiGG ID | Not Available |
|---|
| BioCyc ID | Not Available |
|---|
| METLIN ID | Not Available |
|---|
| PDB ID | Not Available |
|---|
| Wikipedia Link | Diclofenac |
|---|
| Chemspider ID | Not Available |
|---|
| ChEBI ID | 4509 |
|---|
| PubChem Compound ID | 27194 |
|---|
| Kegg Compound ID | Not Available |
|---|
| YMDB ID | Not Available |
|---|
| ECMDB ID | Not Available |
|---|
| References |
|---|
| Synthesis Reference | Not Available |
|---|
| MSDS | Not Available |
|---|
| General References | |
|---|