| Record Information |
|---|
| Version | 1.0 |
|---|
| Creation Date | 2016-05-19 01:58:54 UTC |
|---|
| Update Date | 2016-11-09 01:09:29 UTC |
|---|
| Accession Number | CHEM005172 |
|---|
| Identification |
|---|
| Common Name | CITRONELLYL PHENYLACETATE |
|---|
| Class | Small Molecule |
|---|
| Description | Citronellyl alpha-toluate is a flavouring ingredient and flavour enhancer for fruit flavours. Not reported in nature. |
|---|
| Contaminant Sources | - EAFUS Chemicals
- FooDB Chemicals
|
|---|
| Contaminant Type | Not Available |
|---|
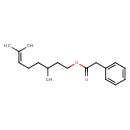
| Chemical Structure | |
|---|
| Synonyms | | Value | Source |
|---|
| Citronellyl a-toluate | Generator | | Citronellyl a-toluic acid | Generator | | Citronellyl alpha-toluic acid | Generator | | Citronellyl α-toluate | Generator | | Citronellyl α-toluic acid | Generator | | 3,7-Dimethyl-6-octen-1-yl phenylacetate | HMDB | | 3,7-Dimethyl-6-octenyl benzeneacetate | HMDB | | 3,7-Dimethyl-6-octenyl phenylacetate | HMDB | | Acetic acid, phenyl-, 3,7-dimethyl-6-octenyl ester | HMDB | | Benzeneacetic acid, 3,7-dimethyl-6-octen-1-yl ester | HMDB | | Benzeneacetic acid, 3,7-dimethyl-6-octenyl ester | HMDB | | Citronellyl phenylacetate | HMDB | | e 2157 | HMDB | | FEMA 2315 | HMDB |
|
|---|
| Chemical Formula | C18H26O2 |
|---|
| Average Molecular Mass | 274.398 g/mol |
|---|
| Monoisotopic Mass | 274.193 g/mol |
|---|
| CAS Registry Number | 139-70-8 |
|---|
| IUPAC Name | 3,7-dimethyloct-6-en-1-yl 2-phenylacetate |
|---|
| Traditional Name | 3,7-dimethyloct-6-en-1-yl 2-phenylacetate |
|---|
| SMILES | CC(CCOC(=O)CC1=CC=CC=C1)CCC=C(C)C |
|---|
| InChI Identifier | InChI=1S/C18H26O2/c1-15(2)8-7-9-16(3)12-13-20-18(19)14-17-10-5-4-6-11-17/h4-6,8,10-11,16H,7,9,12-14H2,1-3H3 |
|---|
| InChI Key | CVJBFMVLVJZZMM-UHFFFAOYSA-N |
|---|
| Chemical Taxonomy |
|---|
| Description | belongs to the class of organic compounds known as fatty alcohol esters. These are ester derivatives of a fatty alcohol. |
|---|
| Kingdom | Organic compounds |
|---|
| Super Class | Lipids and lipid-like molecules |
|---|
| Class | Fatty Acyls |
|---|
| Sub Class | Fatty alcohol esters |
|---|
| Direct Parent | Fatty alcohol esters |
|---|
| Alternative Parents | |
|---|
| Substituents | - Fatty alcohol ester
- Monoterpenoid
- Monocyclic monoterpenoid
- Aromatic monoterpenoid
- Benzenoid
- Monocyclic benzene moiety
- Carboxylic acid ester
- Monocarboxylic acid or derivatives
- Carboxylic acid derivative
- Organic oxygen compound
- Organic oxide
- Hydrocarbon derivative
- Organooxygen compound
- Carbonyl group
- Aromatic homomonocyclic compound
|
|---|
| Molecular Framework | Aromatic homomonocyclic compounds |
|---|
| External Descriptors | Not Available |
|---|
| Biological Properties |
|---|
| Status | Detected and Not Quantified |
|---|
| Origin | Not Available |
|---|
| Cellular Locations | Not Available |
|---|
| Biofluid Locations | Not Available |
|---|
| Tissue Locations | Not Available |
|---|
| Pathways | Not Available |
|---|
| Applications | Not Available |
|---|
| Biological Roles | Not Available |
|---|
| Chemical Roles | Not Available |
|---|
| Physical Properties |
|---|
| State | Not Available |
|---|
| Appearance | Not Available |
|---|
| Experimental Properties | | Property | Value |
|---|
| Melting Point | Not Available | | Boiling Point | Not Available | | Solubility | Not Available |
|
|---|
| Predicted Properties | |
|---|
| Spectra |
|---|
| Spectra | | Spectrum Type | Description | Splash Key | View |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | splash10-0006-9200000000-073702b258e1adfe291b | Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | Not Available | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-004i-2790000000-b80b9ebf5d1e9988ae87 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-014r-5910000000-59459f13b0171cad74f2 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-014l-9200000000-d3285050c4c3bcf49baf | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-00xr-1960000000-3cc848e5dff32cce0761 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-00kr-1910000000-91f60ec045db0c03d31e | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-014u-5900000000-dbca8249a000b0f9eecc | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-00di-0190000000-f2c2f8f406e6a5f92302 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-006x-9650000000-e1f54325e46d4d355368 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-0006-9300000000-088064f5b4177c2b53fc | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-00o9-9740000000-a67826ffcf76a8757933 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-014l-9500000000-29d93ea0e54c4efc5f29 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-0006-9200000000-f9e0b64e0f46a9afadfd | Spectrum |
|
|---|
| Toxicity Profile |
|---|
| Route of Exposure | Not Available |
|---|
| Mechanism of Toxicity | Not Available |
|---|
| Metabolism | Not Available |
|---|
| Toxicity Values | Not Available |
|---|
| Lethal Dose | Not Available |
|---|
| Carcinogenicity (IARC Classification) | Not Available |
|---|
| Uses/Sources | Not Available |
|---|
| Minimum Risk Level | Not Available |
|---|
| Health Effects | Not Available |
|---|
| Symptoms | Not Available |
|---|
| Treatment | Not Available |
|---|
| Concentrations |
|---|
| Not Available |
|---|
| External Links |
|---|
| DrugBank ID | Not Available |
|---|
| HMDB ID | HMDB0037230 |
|---|
| FooDB ID | FDB016237 |
|---|
| Phenol Explorer ID | Not Available |
|---|
| KNApSAcK ID | Not Available |
|---|
| BiGG ID | Not Available |
|---|
| BioCyc ID | Not Available |
|---|
| METLIN ID | Not Available |
|---|
| PDB ID | Not Available |
|---|
| Wikipedia Link | Not Available |
|---|
| Chemspider ID | 8437 |
|---|
| ChEBI ID | Not Available |
|---|
| PubChem Compound ID | 8767 |
|---|
| Kegg Compound ID | Not Available |
|---|
| YMDB ID | Not Available |
|---|
| ECMDB ID | Not Available |
|---|
| References |
|---|
| Synthesis Reference | Not Available |
|---|
| MSDS | Not Available |
|---|
| General References | |
|---|