| Record Information |
|---|
| Version | 1.0 |
|---|
| Creation Date | 2016-05-19 01:58:45 UTC |
|---|
| Update Date | 2016-11-09 01:09:29 UTC |
|---|
| Accession Number | CHEM005161 |
|---|
| Identification |
|---|
| Common Name | CITRAL PROPYLENE GLYCOL ACETAL |
|---|
| Class | Small Molecule |
|---|
| Description | Citral propylene glycol acetal is a flavouring ingredient. |
|---|
| Contaminant Sources | - EAFUS Chemicals
- FooDB Chemicals
|
|---|
| Contaminant Type | Not Available |
|---|
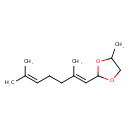
| Chemical Structure | |
|---|
| Synonyms | | Value | Source |
|---|
| 2-(2,6-Dimethyl-1,5-heptadienyl)-4-methyl-1,3-dioxolane | HMDB | | 2-(2,6-Dimethyl-1,5-heptadienyl-4-methyl-1,3-dioxolane | HMDB | | 2-(2,6-Dimethylhepta-1,5-dienyl)-4-methyl-1,3-dioxolane | HMDB | | Citral propyleneglycol acetal | HMDB | | Citral, 1,2-propylene glycol acetal | HMDB |
|
|---|
| Chemical Formula | C13H22O2 |
|---|
| Average Molecular Mass | 210.313 g/mol |
|---|
| Monoisotopic Mass | 210.162 g/mol |
|---|
| CAS Registry Number | 10444-50-5 |
|---|
| IUPAC Name | 2-[(1E)-2,6-dimethylhepta-1,5-dien-1-yl]-4-methyl-1,3-dioxolane |
|---|
| Traditional Name | 2-[(1E)-2,6-dimethylhepta-1,5-dien-1-yl]-4-methyl-1,3-dioxolane |
|---|
| SMILES | CC1COC(O1)\C=C(/C)CCC=C(C)C |
|---|
| InChI Identifier | InChI=1S/C13H22O2/c1-10(2)6-5-7-11(3)8-13-14-9-12(4)15-13/h6,8,12-13H,5,7,9H2,1-4H3/b11-8+ |
|---|
| InChI Key | DWZRENGNFQNWQZ-DHZHZOJOSA-N |
|---|
| Chemical Taxonomy |
|---|
| Description | belongs to the class of organic compounds known as 1,3-dioxolanes. These are organic compounds containing 1,3-dioxolane, an aliphatic five-member ring with two oxygen atoms in ring positions 1 and 3. |
|---|
| Kingdom | Organic compounds |
|---|
| Super Class | Organoheterocyclic compounds |
|---|
| Class | Dioxolanes |
|---|
| Sub Class | 1,3-dioxolanes |
|---|
| Direct Parent | 1,3-dioxolanes |
|---|
| Alternative Parents | |
|---|
| Substituents | - Meta-dioxolane
- Oxacycle
- Acetal
- Organic oxygen compound
- Hydrocarbon derivative
- Organooxygen compound
- Aliphatic heteromonocyclic compound
|
|---|
| Molecular Framework | Aliphatic heteromonocyclic compounds |
|---|
| External Descriptors | Not Available |
|---|
| Biological Properties |
|---|
| Status | Detected and Not Quantified |
|---|
| Origin | Not Available |
|---|
| Cellular Locations | Not Available |
|---|
| Biofluid Locations | Not Available |
|---|
| Tissue Locations | Not Available |
|---|
| Pathways | Not Available |
|---|
| Applications | Not Available |
|---|
| Biological Roles | Not Available |
|---|
| Chemical Roles | Not Available |
|---|
| Physical Properties |
|---|
| State | Not Available |
|---|
| Appearance | Not Available |
|---|
| Experimental Properties | | Property | Value |
|---|
| Melting Point | Not Available | | Boiling Point | Not Available | | Solubility | Not Available |
|
|---|
| Predicted Properties | |
|---|
| Spectra |
|---|
| Spectra | | Spectrum Type | Description | Splash Key | View |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | splash10-05oy-9600000000-bd1799126ba195ec66cf | Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | Not Available | Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | Not Available | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-03di-2970000000-10aaf2c60c59dd3f6a67 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-0cdi-4910000000-fa12956387416e882f88 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-014i-9200000000-d938b63f43ec9dbd4e74 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-0a4i-0090000000-c0b1c2cef3dc0ef5ccf8 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-0a4i-9870000000-aa839686fbd640c73f4a | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-0mbi-3900000000-6faa264b513629df0acc | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-0a4i-0590000000-7d5fe92d644559701ca5 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-05fr-2920000000-e7f08caa23aaca2c7d63 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-054o-9800000000-7b1f7ce479fd181918f5 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-03di-7960000000-3bde298e6c9458cd4ebd | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-05o3-9200000000-5660a891a3cd64134187 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-052f-9100000000-90570bd5da089952d7f3 | Spectrum |
|
|---|
| Toxicity Profile |
|---|
| Route of Exposure | Not Available |
|---|
| Mechanism of Toxicity | Not Available |
|---|
| Metabolism | Not Available |
|---|
| Toxicity Values | Not Available |
|---|
| Lethal Dose | Not Available |
|---|
| Carcinogenicity (IARC Classification) | Not Available |
|---|
| Uses/Sources | Not Available |
|---|
| Minimum Risk Level | Not Available |
|---|
| Health Effects | Not Available |
|---|
| Symptoms | Not Available |
|---|
| Treatment | Not Available |
|---|
| Concentrations |
|---|
| Not Available |
|---|
| External Links |
|---|
| DrugBank ID | Not Available |
|---|
| HMDB ID | HMDB0037286 |
|---|
| FooDB ID | FDB016305 |
|---|
| Phenol Explorer ID | Not Available |
|---|
| KNApSAcK ID | Not Available |
|---|
| BiGG ID | Not Available |
|---|
| BioCyc ID | Not Available |
|---|
| METLIN ID | Not Available |
|---|
| PDB ID | Not Available |
|---|
| Wikipedia Link | Not Available |
|---|
| Chemspider ID | 4495577 |
|---|
| ChEBI ID | Not Available |
|---|
| PubChem Compound ID | 5338463 |
|---|
| Kegg Compound ID | Not Available |
|---|
| YMDB ID | Not Available |
|---|
| ECMDB ID | Not Available |
|---|
| References |
|---|
| Synthesis Reference | Not Available |
|---|
| MSDS | Not Available |
|---|
| General References | |
|---|