| Record Information |
|---|
| Version | 1.0 |
|---|
| Creation Date | 2016-05-19 01:58:16 UTC |
|---|
| Update Date | 2016-11-09 01:09:29 UTC |
|---|
| Accession Number | CHEM005116 |
|---|
| Identification |
|---|
| Common Name | CINNAMALDEHYDE ETHYLENE GLYCOL ACETAL |
|---|
| Class | Small Molecule |
|---|
| Description | Protected form of cinnamaldehyde which liberates cinnamaldehyde flavour on oral contact. 2-(Phenylethenyl)-1,3-dioxolane is used in products such as chewing gum. |
|---|
| Contaminant Sources | - EAFUS Chemicals
- FooDB Chemicals
|
|---|
| Contaminant Type | Not Available |
|---|
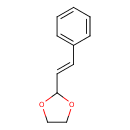
| Chemical Structure | |
|---|
| Synonyms | | Value | Source |
|---|
| 2-(2-Phenylethenyl)-1,3-dioxolane | HMDB | | 2-Styryl-1,3-dioxolane | HMDB | | 2-Styryl-1,3-dioxolane, 8ci | HMDB | | Cinnamaldehyde ethylene acetal | HMDB | | Cinnamaldehyde ethylene glycol acetal | HMDB | | Cinnamaldehyde, cyclic ethylene acetal | HMDB | | Cinncloval | HMDB | | FEMA 2287 | HMDB |
|
|---|
| Chemical Formula | C11H12O2 |
|---|
| Average Molecular Mass | 176.212 g/mol |
|---|
| Monoisotopic Mass | 176.084 g/mol |
|---|
| CAS Registry Number | 5660-60-6 |
|---|
| IUPAC Name | 2-[(E)-2-phenylethenyl]-1,3-dioxolane |
|---|
| Traditional Name | 2-[(E)-2-phenylethenyl]-1,3-dioxolane |
|---|
| SMILES | C1COC(O1)\C=C\C1=CC=CC=C1 |
|---|
| InChI Identifier | InChI=1S/C11H12O2/c1-2-4-10(5-3-1)6-7-11-12-8-9-13-11/h1-7,11H,8-9H2/b7-6+ |
|---|
| InChI Key | JQLASNFFJHGQTK-VOTSOKGWSA-N |
|---|
| Chemical Taxonomy |
|---|
| Description | belongs to the class of organic compounds known as styrenes. These are organic compounds containing an ethenylbenzene moiety. |
|---|
| Kingdom | Organic compounds |
|---|
| Super Class | Benzenoids |
|---|
| Class | Benzene and substituted derivatives |
|---|
| Sub Class | Styrenes |
|---|
| Direct Parent | Styrenes |
|---|
| Alternative Parents | |
|---|
| Substituents | - Styrene
- Meta-dioxolane
- Oxacycle
- Organoheterocyclic compound
- Acetal
- Organic oxygen compound
- Hydrocarbon derivative
- Organooxygen compound
- Aromatic heteromonocyclic compound
|
|---|
| Molecular Framework | Aromatic heteromonocyclic compounds |
|---|
| External Descriptors | Not Available |
|---|
| Biological Properties |
|---|
| Status | Detected and Not Quantified |
|---|
| Origin | Not Available |
|---|
| Cellular Locations | Not Available |
|---|
| Biofluid Locations | Not Available |
|---|
| Tissue Locations | Not Available |
|---|
| Pathways | Not Available |
|---|
| Applications | Not Available |
|---|
| Biological Roles | Not Available |
|---|
| Chemical Roles | Not Available |
|---|
| Physical Properties |
|---|
| State | Not Available |
|---|
| Appearance | Not Available |
|---|
| Experimental Properties | | Property | Value |
|---|
| Melting Point | Not Available | | Boiling Point | Not Available | | Solubility | Not Available |
|
|---|
| Predicted Properties | |
|---|
| Spectra |
|---|
| Spectra | | Spectrum Type | Description | Splash Key | View |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | splash10-00c3-9800000000-6c95597fd1a292e56187 | Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | Not Available | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-004i-2900000000-fecd20c6447e58ffe2e2 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-004i-5900000000-5c2977c5a958c23b57ef | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-0uxu-9800000000-e6f9c48a19124778e08f | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-004i-0900000000-ff300b323328b8dddcfa | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-004i-6900000000-60c3ab2b1eb494ba74bd | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-0f76-7900000000-ae607a0ee1bba395a273 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-004i-1900000000-4ed47831c43e7430353e | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-014i-1900000000-e014175ae179db6d83ea | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-014i-6900000000-979b5885d19d02e858cf | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-0ufr-0900000000-810e4ef1066bae3b4f33 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-014i-1900000000-05d0dc745645059bfe7a | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-004i-9600000000-caae49c039bdba60a8c0 | Spectrum |
|
|---|
| Toxicity Profile |
|---|
| Route of Exposure | Not Available |
|---|
| Mechanism of Toxicity | Not Available |
|---|
| Metabolism | Not Available |
|---|
| Toxicity Values | Not Available |
|---|
| Lethal Dose | Not Available |
|---|
| Carcinogenicity (IARC Classification) | Not Available |
|---|
| Uses/Sources | Not Available |
|---|
| Minimum Risk Level | Not Available |
|---|
| Health Effects | Not Available |
|---|
| Symptoms | Not Available |
|---|
| Treatment | Not Available |
|---|
| Concentrations |
|---|
| Not Available |
|---|
| External Links |
|---|
| DrugBank ID | Not Available |
|---|
| HMDB ID | HMDB0037142 |
|---|
| FooDB ID | FDB016137 |
|---|
| Phenol Explorer ID | Not Available |
|---|
| KNApSAcK ID | Not Available |
|---|
| BiGG ID | Not Available |
|---|
| BioCyc ID | Not Available |
|---|
| METLIN ID | Not Available |
|---|
| PDB ID | Not Available |
|---|
| Wikipedia Link | Not Available |
|---|
| Chemspider ID | 4872367 |
|---|
| ChEBI ID | Not Available |
|---|
| PubChem Compound ID | 6284401 |
|---|
| Kegg Compound ID | Not Available |
|---|
| YMDB ID | Not Available |
|---|
| ECMDB ID | Not Available |
|---|
| References |
|---|
| Synthesis Reference | Not Available |
|---|
| MSDS | Not Available |
|---|
| General References | |
|---|