| Record Information |
|---|
| Version | 1.0 |
|---|
| Creation Date | 2016-05-19 01:58:10 UTC |
|---|
| Update Date | 2016-11-09 01:09:29 UTC |
|---|
| Accession Number | CHEM005104 |
|---|
| Identification |
|---|
| Common Name | CHOLINE CHLORIDE |
|---|
| Class | Small Molecule |
|---|
| Description | A quaternary ammonium salt with choline cation and chloride anion. |
|---|
| Contaminant Sources | - EAFUS Chemicals
- FooDB Chemicals
- HPV EPA Chemicals
- OECD HPV Chemicals
- STOFF IDENT Compounds
- ToxCast & Tox21 Chemicals
|
|---|
| Contaminant Type | Not Available |
|---|
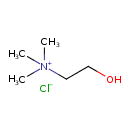
| Chemical Structure | |
|---|
| Synonyms | | Value | Source |
|---|
| (2-Hydroxyethyl)trimethylammonium chloride | ChEBI | | (beta-Hydroxyethyl)trimethylammonium chloride | ChEBI | | 2-Hydroxy-N,N,N,-trimethylethanaminium chloride | ChEBI | | Bilineurin chloride | ChEBI | | Biocolina | ChEBI | | Biocoline | ChEBI | | Chloride de choline | ChEBI | | Chlorure de choline | ChEBI | | Choline chlorhydrate | ChEBI | | Choline hydrochloride | ChEBI | | Cholini chloridum | ChEBI | | Cholinium chloride | ChEBI | | Cloruro de colina | ChEBI | | Hepacholine | ChEBI | | Lipotril | ChEBI | | Luridin chloride | ChEBI | | Paresan | ChEBI | | Trimethyl(2-hydroxyethyl)ammonium chloride | ChEBI | | (b-Hydroxyethyl)trimethylammonium chloride | Generator | | (Β-hydroxyethyl)trimethylammonium chloride | Generator | | Choline chlorhydric acid | Generator | | Choline O-sulfate | MeSH | | Citrate, choline | MeSH | | Fagine | MeSH | | Hydroxide, choline | MeSH | | Vidine | MeSH | | Choline bitartrate | MeSH | | Choline hydroxide | MeSH | | Choline O sulfate | MeSH | | O-Sulfate, choline | MeSH | | 2-Hydroxy-N,N,N-trimethylethanaminium | MeSH | | Choline | MeSH | | Bitartrate, choline | MeSH | | Bursine | MeSH | | Chloride, choline | MeSH | | Choline citrate | MeSH |
|
|---|
| Chemical Formula | C5H14ClNO |
|---|
| Average Molecular Mass | 139.624 g/mol |
|---|
| Monoisotopic Mass | 139.076 g/mol |
|---|
| CAS Registry Number | 67-48-1 |
|---|
| IUPAC Name | (2-hydroxyethyl)trimethylazanium chloride |
|---|
| Traditional Name | choline chloride |
|---|
| SMILES | [Cl-].C[N+](C)(C)CCO |
|---|
| InChI Identifier | InChI=1S/C5H14NO.ClH/c1-6(2,3)4-5-7;/h7H,4-5H2,1-3H3;1H/q+1;/p-1 |
|---|
| InChI Key | SGMZJAMFUVOLNK-UHFFFAOYSA-M |
|---|
| Chemical Taxonomy |
|---|
| Description | belongs to the class of organic compounds known as cholines. These are organic compounds containing a N,N,N-trimethylethanolammonium cation. |
|---|
| Kingdom | Organic compounds |
|---|
| Super Class | Organic nitrogen compounds |
|---|
| Class | Organonitrogen compounds |
|---|
| Sub Class | Quaternary ammonium salts |
|---|
| Direct Parent | Cholines |
|---|
| Alternative Parents | |
|---|
| Substituents | - Choline
- Tetraalkylammonium salt
- 1,2-aminoalcohol
- Alkanolamine
- Organic oxygen compound
- Organopnictogen compound
- Hydrocarbon derivative
- Organic chloride salt
- Organic salt
- Organic zwitterion
- Primary alcohol
- Organooxygen compound
- Amine
- Alcohol
- Aliphatic acyclic compound
|
|---|
| Molecular Framework | Aliphatic acyclic compounds |
|---|
| External Descriptors | |
|---|
| Biological Properties |
|---|
| Status | Detected and Not Quantified |
|---|
| Origin | Not Available |
|---|
| Cellular Locations | Not Available |
|---|
| Biofluid Locations | Not Available |
|---|
| Tissue Locations | Not Available |
|---|
| Pathways | Not Available |
|---|
| Applications | Not Available |
|---|
| Biological Roles | Not Available |
|---|
| Chemical Roles | Not Available |
|---|
| Physical Properties |
|---|
| State | Not Available |
|---|
| Appearance | Not Available |
|---|
| Experimental Properties | | Property | Value |
|---|
| Melting Point | Not Available | | Boiling Point | Not Available | | Solubility | Not Available |
|
|---|
| Predicted Properties | |
|---|
| Spectra |
|---|
| Spectra | | Spectrum Type | Description | Splash Key | View |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | splash10-0ab9-6900000000-b8ca467226de252852cf | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-0006-1900000000-8874de5148aca4d047fc | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-00du-4900000000-95cc5e5bb585cf1f6c92 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-00di-8900000000-6f4987e8e48b063ffb68 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-000i-0900000000-9da59579bff9834322d1 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-0570-5900000000-1ef34bebbd29541214a9 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-0059-9100000000-acbccf691e21dcbabc6a | Spectrum |
|
|---|
| Toxicity Profile |
|---|
| Route of Exposure | Not Available |
|---|
| Mechanism of Toxicity | Not Available |
|---|
| Metabolism | Not Available |
|---|
| Toxicity Values | Not Available |
|---|
| Lethal Dose | Not Available |
|---|
| Carcinogenicity (IARC Classification) | Not Available |
|---|
| Uses/Sources | Not Available |
|---|
| Minimum Risk Level | Not Available |
|---|
| Health Effects | Not Available |
|---|
| Symptoms | Not Available |
|---|
| Treatment | Not Available |
|---|
| Concentrations |
|---|
| Not Available |
|---|
| External Links |
|---|
| DrugBank ID | Not Available |
|---|
| HMDB ID | HMDB0029558 |
|---|
| FooDB ID | FDB000711 |
|---|
| Phenol Explorer ID | Not Available |
|---|
| KNApSAcK ID | Not Available |
|---|
| BiGG ID | Not Available |
|---|
| BioCyc ID | Not Available |
|---|
| METLIN ID | Not Available |
|---|
| PDB ID | Not Available |
|---|
| Wikipedia Link | Choline_chloride |
|---|
| Chemspider ID | Not Available |
|---|
| ChEBI ID | 133341 |
|---|
| PubChem Compound ID | 6209 |
|---|
| Kegg Compound ID | Not Available |
|---|
| YMDB ID | Not Available |
|---|
| ECMDB ID | Not Available |
|---|
| References |
|---|
| Synthesis Reference | Not Available |
|---|
| MSDS | Not Available |
|---|
| General References | |
|---|