| Record Information |
|---|
| Version | 1.0 |
|---|
| Creation Date | 2016-05-19 01:57:33 UTC |
|---|
| Update Date | 2016-11-09 01:09:28 UTC |
|---|
| Accession Number | CHEM005038 |
|---|
| Identification |
|---|
| Common Name | BETA-CARYOPHYLLENE ALCOHOL |
|---|
| Class | Small Molecule |
|---|
| Description | beta-Caryophyllene alcohol is found in citrus. beta-Caryophyllene alcohol is a constituent of grapefruit juice, pimento berry, Korean chamchwi and the essential oils of Palmarosa, peppermint, clove, hop, pepper, bergamot and Sicilian sumac fruit (Rhus coriaria). beta-Caryophyllene alcohol is a flavouring ingredient either alone or together with a-Caryophyllene alcohol KFS99-I. |
|---|
| Contaminant Sources | - EAFUS Chemicals
- FooDB Chemicals
|
|---|
| Contaminant Type | Not Available |
|---|
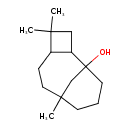
| Chemical Structure | |
|---|
| Synonyms | | Value | Source |
|---|
| b-Caryophyllene alcohol | Generator | | Β-caryophyllene alcohol | Generator | | 4,4,8-Trimethyltricyclo(6.3.1.02,5)dodecan-1-ol | MeSH | | Caryolan-1-ol | MeSH | | 4,4,8-Trimethyl-tricyclo(6.3.1.02,5)dodecan-1-ol | HMDB | | 4,4,8-trimethyltricyclo[6.3.1.02,5]Dodecan-1-ol, 9ci | HMDB | | Caryophyllenol | HMDB | | beta-Caryophyllene alcohol | MeSH |
|
|---|
| Chemical Formula | C15H26O |
|---|
| Average Molecular Mass | 222.366 g/mol |
|---|
| Monoisotopic Mass | 222.198 g/mol |
|---|
| CAS Registry Number | 472-97-9 |
|---|
| IUPAC Name | 4,4,8-trimethyltricyclo[6.3.1.0²,⁵]dodecan-1-ol |
|---|
| Traditional Name | 4,4,8-trimethyltricyclo[6.3.1.0²,⁵]dodecan-1-ol |
|---|
| SMILES | CC1(C)CC2C1CCC1(C)CCCC2(O)C1 |
|---|
| InChI Identifier | InChI=1S/C15H26O/c1-13(2)9-12-11(13)5-8-14(3)6-4-7-15(12,16)10-14/h11-12,16H,4-10H2,1-3H3 |
|---|
| InChI Key | FUQAYSQLAOJBBC-UHFFFAOYSA-N |
|---|
| Chemical Taxonomy |
|---|
| Description | belongs to the class of organic compounds known as tertiary alcohols. Tertiary alcohols are compounds in which a hydroxy group, -OH, is attached to a saturated carbon atom R3COH (R not H ). |
|---|
| Kingdom | Organic compounds |
|---|
| Super Class | Organic oxygen compounds |
|---|
| Class | Organooxygen compounds |
|---|
| Sub Class | Alcohols and polyols |
|---|
| Direct Parent | Tertiary alcohols |
|---|
| Alternative Parents | |
|---|
| Substituents | - Tertiary alcohol
- Cyclic alcohol
- Hydrocarbon derivative
- Aliphatic homopolycyclic compound
|
|---|
| Molecular Framework | Aliphatic homopolycyclic compounds |
|---|
| External Descriptors | |
|---|
| Biological Properties |
|---|
| Status | Detected and Not Quantified |
|---|
| Origin | Not Available |
|---|
| Cellular Locations | Not Available |
|---|
| Biofluid Locations | Not Available |
|---|
| Tissue Locations | Not Available |
|---|
| Pathways | Not Available |
|---|
| Applications | Not Available |
|---|
| Biological Roles | Not Available |
|---|
| Chemical Roles | Not Available |
|---|
| Physical Properties |
|---|
| State | Not Available |
|---|
| Appearance | Not Available |
|---|
| Experimental Properties | | Property | Value |
|---|
| Melting Point | Not Available | | Boiling Point | Not Available | | Solubility | Not Available |
|
|---|
| Predicted Properties | |
|---|
| Spectra |
|---|
| Spectra | | Spectrum Type | Description | Splash Key | View |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | splash10-06tf-3920000000-b68753a0c64ca8112abc | Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (1 TMS) - 70eV, Positive | splash10-0v7r-5290000000-2abb69265dc4b41e9d83 | Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | Not Available | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-0ab9-0090000000-f722d04da4e967382a9b | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-0ab9-1290000000-68742d2f6dacdd03ba4c | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-054x-8910000000-6d5494cbf7239c6a6288 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-00di-0090000000-df53e329d8b4cb321660 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-00di-0090000000-78f3f8caa5d303c302f1 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-0a4i-3950000000-68721e6a300ef023c3c0 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-00di-0090000000-ab61d8a2af41e6ed2e49 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-00di-0090000000-ab61d8a2af41e6ed2e49 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-00di-0090000000-ab61d8a2af41e6ed2e49 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-00di-1290000000-b8ad223195b98618f733 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-0ac0-9660000000-c0162b1be56a39c1cd11 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-00l6-9100000000-768051f4547352e86ab4 | Spectrum |
|
|---|
| Toxicity Profile |
|---|
| Route of Exposure | Not Available |
|---|
| Mechanism of Toxicity | Not Available |
|---|
| Metabolism | Not Available |
|---|
| Toxicity Values | Not Available |
|---|
| Lethal Dose | Not Available |
|---|
| Carcinogenicity (IARC Classification) | Not Available |
|---|
| Uses/Sources | Not Available |
|---|
| Minimum Risk Level | Not Available |
|---|
| Health Effects | Not Available |
|---|
| Symptoms | Not Available |
|---|
| Treatment | Not Available |
|---|
| Concentrations |
|---|
| Not Available |
|---|
| External Links |
|---|
| DrugBank ID | Not Available |
|---|
| HMDB ID | HMDB0037066 |
|---|
| FooDB ID | FDB016050 |
|---|
| Phenol Explorer ID | Not Available |
|---|
| KNApSAcK ID | C00021969 |
|---|
| BiGG ID | Not Available |
|---|
| BioCyc ID | Not Available |
|---|
| METLIN ID | Not Available |
|---|
| PDB ID | Not Available |
|---|
| Wikipedia Link | Not Available |
|---|
| Chemspider ID | 55073 |
|---|
| ChEBI ID | Not Available |
|---|
| PubChem Compound ID | 61125 |
|---|
| Kegg Compound ID | Not Available |
|---|
| YMDB ID | Not Available |
|---|
| ECMDB ID | Not Available |
|---|
| References |
|---|
| Synthesis Reference | Not Available |
|---|
| MSDS | Not Available |
|---|
| General References | |
|---|