| Record Information |
|---|
| Version | 1.0 |
|---|
| Creation Date | 2016-05-19 01:57:30 UTC |
|---|
| Update Date | 2016-11-09 01:09:28 UTC |
|---|
| Accession Number | CHEM005034 |
|---|
| Identification |
|---|
| Common Name | CARVYL ACETATE |
|---|
| Class | Small Molecule |
|---|
| Description | Carvyl acetate is found in caraway. Carvyl acetate is a flavouring ingredient. |
|---|
| Contaminant Sources | - EAFUS Chemicals
- FooDB Chemicals
- ToxCast & Tox21 Chemicals
|
|---|
| Contaminant Type | Not Available |
|---|
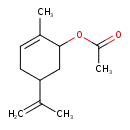
| Chemical Structure | |
|---|
| Synonyms | | Value | Source |
|---|
| Carvyl acetic acid | Generator | | 1-P-Mentha-6(8,9)-dien-2-yl acetate | HMDB | | 2-Cyclohexen-1-ol, 2-methyl-5-(1-methylethenyl)-, 1-acetate | HMDB | | 2-Cyclohexen-1-ol, 2-methyl-5-(1-methylethenyl)-, acetate | HMDB | | 2-Methyl-5-(1-methylethenyl)-2-cyclohexen-1-yl acetate | HMDB | | 5-Isopropenyl-2-methyl-2-cyclohexen-1-yl acetate | HMDB | | 5-Isopropenyl-2-methylcyclohex-2-en-1-yl acetate | HMDB | | 6-Acetoxy-P-menta-1,8-diene | HMDB | | Carveol acetate | HMDB | | FEMA 2250 | HMDB | | P-Mentha-1(6),8-dien-2-yl acetate | HMDB | | P-Mentha-6,8-dien-2-ol, acetate | HMDB | | 2-Methyl-5-(prop-1-en-2-yl)cyclohex-2-en-1-yl acetic acid | Generator |
|
|---|
| Chemical Formula | C12H18O2 |
|---|
| Average Molecular Mass | 194.270 g/mol |
|---|
| Monoisotopic Mass | 194.131 g/mol |
|---|
| CAS Registry Number | 97-42-7 |
|---|
| IUPAC Name | 2-methyl-5-(prop-1-en-2-yl)cyclohex-2-en-1-yl acetate |
|---|
| Traditional Name | 2-methyl-5-(prop-1-en-2-yl)cyclohex-2-en-1-yl acetate |
|---|
| SMILES | CC(=O)OC1CC(CC=C1C)C(C)=C |
|---|
| InChI Identifier | InChI=1S/C12H18O2/c1-8(2)11-6-5-9(3)12(7-11)14-10(4)13/h5,11-12H,1,6-7H2,2-4H3 |
|---|
| InChI Key | YTHRBOFHFYZBRJ-UHFFFAOYSA-N |
|---|
| Chemical Taxonomy |
|---|
| Description | belongs to the class of organic compounds known as menthane monoterpenoids. These are monoterpenoids with a structure based on the o-, m-, or p-menthane backbone. P-menthane consists of the cyclohexane ring with a methyl group and a (2-methyl)-propyl group at the 1 and 4 ring position, respectively. The o- and m- menthanes are much rarer, and presumably arise by alkyl migration of p-menthanes. |
|---|
| Kingdom | Organic compounds |
|---|
| Super Class | Lipids and lipid-like molecules |
|---|
| Class | Prenol lipids |
|---|
| Sub Class | Monoterpenoids |
|---|
| Direct Parent | Menthane monoterpenoids |
|---|
| Alternative Parents | |
|---|
| Substituents | - P-menthane monoterpenoid
- Monocyclic monoterpenoid
- Carboxylic acid ester
- Monocarboxylic acid or derivatives
- Carboxylic acid derivative
- Organic oxygen compound
- Organic oxide
- Hydrocarbon derivative
- Organooxygen compound
- Carbonyl group
- Aliphatic homomonocyclic compound
|
|---|
| Molecular Framework | Aliphatic homomonocyclic compounds |
|---|
| External Descriptors | Not Available |
|---|
| Biological Properties |
|---|
| Status | Detected and Not Quantified |
|---|
| Origin | Not Available |
|---|
| Cellular Locations | Not Available |
|---|
| Biofluid Locations | Not Available |
|---|
| Tissue Locations | Not Available |
|---|
| Pathways | Not Available |
|---|
| Applications | Not Available |
|---|
| Biological Roles | Not Available |
|---|
| Chemical Roles | Not Available |
|---|
| Physical Properties |
|---|
| State | Not Available |
|---|
| Appearance | Not Available |
|---|
| Experimental Properties | | Property | Value |
|---|
| Melting Point | Not Available | | Boiling Point | Not Available | | Solubility | Not Available |
|
|---|
| Predicted Properties | |
|---|
| Spectra |
|---|
| Spectra | | Spectrum Type | Description | Splash Key | View |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | splash10-000l-9600000000-09243828e18aa41e0857 | Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | Not Available | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-0002-1900000000-72048022935d06795db5 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-0f7a-4900000000-96b0d2fa5261b994e15d | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-0uxr-9200000000-b5f95b7ac1c0802136b2 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-0f6x-1900000000-a349e46456c32b928cdd | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-0udl-2900000000-2e37cdade8742dccbd7b | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-0k9l-5900000000-7d5a204ab34a0f65036b | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-000b-4900000000-74644adc674fcdd2e2d6 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-0017-9700000000-6e51e856dc751a7f27de | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-0006-9200000000-719c0e6361f4ae486958 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-0k96-3900000000-78b1ea6b982231cc92b9 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-0a4i-9200000000-f85ed24e5b80b4d28e64 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-0a4l-9300000000-eacfac41e8ab4441d467 | Spectrum |
|
|---|
| Toxicity Profile |
|---|
| Route of Exposure | Not Available |
|---|
| Mechanism of Toxicity | Not Available |
|---|
| Metabolism | Not Available |
|---|
| Toxicity Values | Not Available |
|---|
| Lethal Dose | Not Available |
|---|
| Carcinogenicity (IARC Classification) | Not Available |
|---|
| Uses/Sources | Not Available |
|---|
| Minimum Risk Level | Not Available |
|---|
| Health Effects | Not Available |
|---|
| Symptoms | Not Available |
|---|
| Treatment | Not Available |
|---|
| Concentrations |
|---|
| Not Available |
|---|
| External Links |
|---|
| DrugBank ID | Not Available |
|---|
| HMDB ID | HMDB0038046 |
|---|
| FooDB ID | FDB017256 |
|---|
| Phenol Explorer ID | Not Available |
|---|
| KNApSAcK ID | C00048350 |
|---|
| BiGG ID | Not Available |
|---|
| BioCyc ID | Not Available |
|---|
| METLIN ID | Not Available |
|---|
| PDB ID | Not Available |
|---|
| Wikipedia Link | Not Available |
|---|
| Chemspider ID | 7058 |
|---|
| ChEBI ID | Not Available |
|---|
| PubChem Compound ID | 7335 |
|---|
| Kegg Compound ID | Not Available |
|---|
| YMDB ID | Not Available |
|---|
| ECMDB ID | Not Available |
|---|
| References |
|---|
| Synthesis Reference | Not Available |
|---|
| MSDS | Not Available |
|---|
| General References | |
|---|