| Record Information |
|---|
| Version | 1.0 |
|---|
| Creation Date | 2016-05-19 01:57:17 UTC |
|---|
| Update Date | 2016-10-28 10:01:53 UTC |
|---|
| Accession Number | CHEM005015 |
|---|
| Identification |
|---|
| Common Name | BETA-CAROTENE |
|---|
| Class | Small Molecule |
|---|
| Description | Beta-Carotene, also known as B-carotene, belongs to the class of organic compounds known as carotenes. These are a type of unsaturated hydrocarbons containing eight consecutive isoprene units. They are characterized by the presence of two end-groups (mostly cyclohexene rings, but also cyclopentene rings or acyclic groups) linked by a long branched alkyl chain. Carotenes belonging form a subgroup of the carotenoids family. Beta-Carotene is possibly soluble (in water) and possibly neutral. Beta-Carotene exists in all living species, ranging from bacteria to humans. |
|---|
| Contaminant Sources | - Cosmetic Chemicals
- EAFUS Chemicals
- FooDB Chemicals
- STOFF IDENT Compounds
- ToxCast & Tox21 Chemicals
|
|---|
| Contaminant Type | Not Available |
|---|
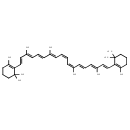
| Chemical Structure | |
|---|
| Synonyms | | Value | Source |
|---|
| b-Carotene | Generator | | Β-carotene | Generator, HMDB |
|
|---|
| Chemical Formula | C40H56 |
|---|
| Average Molecular Mass | 536.888 g/mol |
|---|
| Monoisotopic Mass | 536.438 g/mol |
|---|
| CAS Registry Number | 7235-40-7 |
|---|
| IUPAC Name | 1,3,3-trimethyl-2-[(1E,3E,5E,7E,9Z,11Z,13E,15E,17E)-3,7,12,16-tetramethyl-18-(2,6,6-trimethylcyclohex-1-en-1-yl)octadeca-1,3,5,7,9,11,13,15,17-nonaen-1-yl]cyclohex-1-ene |
|---|
| Traditional Name | 1,3,3-trimethyl-2-[(1E,3E,5E,7E,9Z,11Z,13E,15E,17E)-3,7,12,16-tetramethyl-18-(2,6,6-trimethylcyclohex-1-en-1-yl)octadeca-1,3,5,7,9,11,13,15,17-nonaen-1-yl]cyclohex-1-ene |
|---|
| SMILES | C\C(\C=C\C=C(/C)\C=C\C1=C(C)CCCC1(C)C)=C/C=C/C=C(\C)/C=C/C=C(\C)/C=C/C1=C(C)CCCC1(C)C |
|---|
| InChI Identifier | InChI=1S/C40H56/c1-31(19-13-21-33(3)25-27-37-35(5)23-15-29-39(37,7)8)17-11-12-18-32(2)20-14-22-34(4)26-28-38-36(6)24-16-30-40(38,9)10/h11-14,17-22,25-28H,15-16,23-24,29-30H2,1-10H3/b12-11+,19-13+,20-14+,27-25+,28-26+,31-17+,32-18+,33-21+,34-22+ |
|---|
| InChI Key | OENHQHLEOONYIE-JLTXGRSLSA-N |
|---|
| Chemical Taxonomy |
|---|
| Description | belongs to the class of organic compounds known as carotenes. These are a type of unsaturated hydrocarbons containing eight consecutive isoprene units. They are characterized by the presence of two end-groups (mostly cyclohexene rings, but also cyclopentene rings or acyclic groups) linked by a long branched alkyl chain. Carotenes belonging form a subgroup of the carotenoids family. |
|---|
| Kingdom | Organic compounds |
|---|
| Super Class | Lipids and lipid-like molecules |
|---|
| Class | Prenol lipids |
|---|
| Sub Class | Tetraterpenoids |
|---|
| Direct Parent | Carotenes |
|---|
| Alternative Parents | |
|---|
| Substituents | - Carotene
- Branched unsaturated hydrocarbon
- Cycloalkene
- Cyclic olefin
- Unsaturated aliphatic hydrocarbon
- Unsaturated hydrocarbon
- Olefin
- Hydrocarbon
- Aliphatic homomonocyclic compound
|
|---|
| Molecular Framework | Aliphatic homomonocyclic compounds |
|---|
| External Descriptors | Not Available |
|---|
| Biological Properties |
|---|
| Status | Detected and Not Quantified |
|---|
| Origin | Not Available |
|---|
| Cellular Locations | Not Available |
|---|
| Biofluid Locations | Not Available |
|---|
| Tissue Locations | Not Available |
|---|
| Pathways | Not Available |
|---|
| Applications | Not Available |
|---|
| Biological Roles | Not Available |
|---|
| Chemical Roles | Not Available |
|---|
| Physical Properties |
|---|
| State | Not Available |
|---|
| Appearance | Not Available |
|---|
| Experimental Properties | | Property | Value |
|---|
| Melting Point | Not Available | | Boiling Point | Not Available | | Solubility | Not Available |
|
|---|
| Predicted Properties | |
|---|
| Spectra |
|---|
| Spectra | | Spectrum Type | Description | Splash Key | View |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | splash10-00di-3211290000-f968c686d39ae0f0fc50 | Spectrum | | LC-MS/MS | LC-MS/MS Spectrum - Quattro_QQQ 10V, Positive (Annotated) | splash10-0016-2794670000-c5bc2a4dbcd0f12cbf7f | Spectrum | | LC-MS/MS | LC-MS/MS Spectrum - Quattro_QQQ 25V, Positive (Annotated) | splash10-0002-2687970000-782d048d873c21fc6b82 | Spectrum | | LC-MS/MS | LC-MS/MS Spectrum - Quattro_QQQ 40V, Positive (Annotated) | splash10-05ai-1009510000-585581456b10671692bd | Spectrum | | LC-MS/MS | LC-MS/MS Spectrum - FAB-EBEB (JMS-HX/HX 110A, JEOL) , Positive | splash10-05mx-2920010000-471219f3e66bafb29bee | Spectrum | | LC-MS/MS | LC-MS/MS Spectrum - , positive | splash10-0006-0144900000-2fa7b609c7300ff8aba3 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-000i-0534290000-556febfe6f0feaffd959 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-0012-0967200000-81bf905ffd7943cfc997 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-000j-3869300000-bce59dcd4ea231326003 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-000i-0000090000-300e8e4a326ba007573b | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-000i-0000090000-3ea2a05dae2343b7708f | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-014r-0968170000-f18c08aac9d12ac133d7 | Spectrum | | MS | Mass Spectrum (Electron Ionization) | splash10-05mx-9810010000-9a261fb8602890c2481e | Spectrum | | 2D NMR | [1H,13C] 2D NMR Spectrum | Not Available | Spectrum |
|
|---|
| Toxicity Profile |
|---|
| Route of Exposure | Not Available |
|---|
| Mechanism of Toxicity | Not Available |
|---|
| Metabolism | Not Available |
|---|
| Toxicity Values | Not Available |
|---|
| Lethal Dose | Not Available |
|---|
| Carcinogenicity (IARC Classification) | Not Available |
|---|
| Uses/Sources | Not Available |
|---|
| Minimum Risk Level | Not Available |
|---|
| Health Effects | Not Available |
|---|
| Symptoms | Not Available |
|---|
| Treatment | Not Available |
|---|
| Concentrations |
|---|
| Not Available |
|---|
| External Links |
|---|
| DrugBank ID | Not Available |
|---|
| HMDB ID | HMDB0002935 |
|---|
| FooDB ID | Not Available |
|---|
| Phenol Explorer ID | Not Available |
|---|
| KNApSAcK ID | Not Available |
|---|
| BiGG ID | Not Available |
|---|
| BioCyc ID | Not Available |
|---|
| METLIN ID | Not Available |
|---|
| PDB ID | Not Available |
|---|
| Wikipedia Link | Carotene |
|---|
| Chemspider ID | Not Available |
|---|
| ChEBI ID | Not Available |
|---|
| PubChem Compound ID | 5927371 |
|---|
| Kegg Compound ID | Not Available |
|---|
| YMDB ID | YMDB01515 |
|---|
| ECMDB ID | M2MDB005052 |
|---|
| References |
|---|
| Synthesis Reference | Not Available |
|---|
| MSDS | Not Available |
|---|
| General References | | 1. Larson LL, Wallen SE, Owen FG, Lowry SR: Relation of age, season, production, and health indices to iodine and beta-carotene concentrations in cow's milk. J Dairy Sci. 1983 Dec;66(12):2557-62. doi: 10.3168/jds.S0022-0302(83)82126-2. | | 2. Noziere P, Grolier P, Durand D, Ferlay A, Pradel P, Martin B: Variations in carotenoids, fat-soluble micronutrients, and color in cows' plasma and milk following changes in forage and feeding level. J Dairy Sci. 2006 Jul;89(7):2634-48. doi: 10.3168/jds.S0022-0302(06)72340-2. | | 3. Jensen RG: The composition of bovine milk lipids: January 1995 to December 2000. J Dairy Sci. 2002 Feb;85(2):295-350. doi: 10.3168/jds.S0022-0302(02)74079-4. | | 4. International Dairy Journal; Volume 14, Issue 7, July 2004, Pages 563-570 | | 5. International Dairy Journal; Volume 15, Issue 5, May 2005, Pages 521-526 | | 6. Chotyakul N, Pateiro-Moure M, Saraiva JA, Torres JA, Pérez-Lamela C. 2014. Simultaneous HPLC–DAD quantification of vitamins A and E content in raw, pasteurized, and UHT cow’s milk and their changes during storage. European Food Research and Technology. 238(4):535–547 | | 7. Fooddata+, The Technical University of Denmark (DTU): https://frida.fooddata.dk/QueryFood.php?fn=milk&lang=en |
|
|---|