| Record Information |
|---|
| Version | 1.0 |
|---|
| Creation Date | 2016-05-19 01:56:58 UTC |
|---|
| Update Date | 2016-11-09 01:09:27 UTC |
|---|
| Accession Number | CHEM004979 |
|---|
| Identification |
|---|
| Common Name | CAMPHOLENE ACETATE |
|---|
| Class | Small Molecule |
|---|
| Description | xi-Campholene acetate is found in fruits. xi-Campholene acetate is a flavouring ingredient. alpha-Campholene acetate is a constituent of Juniperus communis (juniper |
|---|
| Contaminant Sources | - EAFUS Chemicals
- FooDB Chemicals
|
|---|
| Contaminant Type | Not Available |
|---|
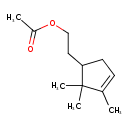
| Chemical Structure | |
|---|
| Synonyms | | Value | Source |
|---|
| a-Campholene acetate | Generator | | a-Campholene acetic acid | Generator | | alpha-Campholene acetic acid | Generator | | Α-campholene acetate | Generator | | Α-campholene acetic acid | Generator | | 1-Acetoxy-2-(2,2,3-trimethyl-3-cyclopentenyl)ethane | HMDB | | 2,2,3-Trimethyl-3-cyclopentene-1-ethanol acetate | HMDB | | 2,2,3-Trimethylcyclopent-3-ene-1-ethyl acetate | HMDB | | 2-(2,2,3-Trimethyl-3-cyclopentenyl)ethyl acetate | HMDB | | 3-Cyclopentene-1-ethanol, 2,2,3-trimethyl-, acetate | HMDB | | alpha-Campholenyl acetate | HMDB | | Campholene acetate | HMDB | | 2-(2,2,3-Trimethylcyclopent-3-en-1-yl)ethyl acetic acid | Generator |
|
|---|
| Chemical Formula | C12H20O2 |
|---|
| Average Molecular Mass | 196.286 g/mol |
|---|
| Monoisotopic Mass | 196.146 g/mol |
|---|
| CAS Registry Number | 1727-68-0 |
|---|
| IUPAC Name | 2-(2,2,3-trimethylcyclopent-3-en-1-yl)ethyl acetate |
|---|
| Traditional Name | 2-(2,2,3-trimethylcyclopent-3-en-1-yl)ethyl acetate |
|---|
| SMILES | CC(=O)OCCC1CC=C(C)C1(C)C |
|---|
| InChI Identifier | InChI=1S/C12H20O2/c1-9-5-6-11(12(9,3)4)7-8-14-10(2)13/h5,11H,6-8H2,1-4H3 |
|---|
| InChI Key | HBRWKAJTMKFEQR-UHFFFAOYSA-N |
|---|
| Chemical Taxonomy |
|---|
| Description | belongs to the class of organic compounds known as monocyclic monoterpenoids. These are monoterpenoids containing 1 ring in the isoprene chain. |
|---|
| Kingdom | Organic compounds |
|---|
| Super Class | Lipids and lipid-like molecules |
|---|
| Class | Prenol lipids |
|---|
| Sub Class | Monoterpenoids |
|---|
| Direct Parent | Monocyclic monoterpenoids |
|---|
| Alternative Parents | |
|---|
| Substituents | - Monocyclic monoterpenoid
- Carboxylic acid ester
- Monocarboxylic acid or derivatives
- Carboxylic acid derivative
- Organic oxygen compound
- Organic oxide
- Hydrocarbon derivative
- Organooxygen compound
- Carbonyl group
- Aliphatic homomonocyclic compound
|
|---|
| Molecular Framework | Aliphatic homomonocyclic compounds |
|---|
| External Descriptors | Not Available |
|---|
| Biological Properties |
|---|
| Status | Detected and Not Quantified |
|---|
| Origin | Not Available |
|---|
| Cellular Locations | Not Available |
|---|
| Biofluid Locations | Not Available |
|---|
| Tissue Locations | Not Available |
|---|
| Pathways | Not Available |
|---|
| Applications | Not Available |
|---|
| Biological Roles | Not Available |
|---|
| Chemical Roles | Not Available |
|---|
| Physical Properties |
|---|
| State | Not Available |
|---|
| Appearance | Not Available |
|---|
| Experimental Properties | | Property | Value |
|---|
| Melting Point | Not Available | | Boiling Point | Not Available | | Solubility | Not Available |
|
|---|
| Predicted Properties | |
|---|
| Spectra |
|---|
| Spectra | | Spectrum Type | Description | Splash Key | View |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | splash10-0a4i-4900000000-807d66676d9ca111f60e | Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | Not Available | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-0002-1900000000-e49a466536d4dd86b7b2 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-000i-4900000000-08f60b252dbda5b4f009 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-0uxu-9100000000-ef26c66adea15f5b8acb | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-0002-3900000000-56739e7a8eae36843fd2 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-0a4i-9400000000-a32d3a469d9df98dae68 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-0a4l-9300000000-ae6e62ea093e89f8325b | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-0a4i-2900000000-9c3ae11c163cd08f3df0 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-052f-9300000000-884f5fa56a780d4ab0de | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-0aou-9600000000-7c51491c39e9daf66749 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-0zfs-3900000000-adc70cb71a3855ca44cb | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-0a4i-9200000000-9988e2f489e1b979d05d | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-0a4l-9000000000-9e44a2b8bb984edc5aaa | Spectrum |
|
|---|
| Toxicity Profile |
|---|
| Route of Exposure | Not Available |
|---|
| Mechanism of Toxicity | Not Available |
|---|
| Metabolism | Not Available |
|---|
| Toxicity Values | Not Available |
|---|
| Lethal Dose | Not Available |
|---|
| Carcinogenicity (IARC Classification) | Not Available |
|---|
| Uses/Sources | Not Available |
|---|
| Minimum Risk Level | Not Available |
|---|
| Health Effects | Not Available |
|---|
| Symptoms | Not Available |
|---|
| Treatment | Not Available |
|---|
| Concentrations |
|---|
| Not Available |
|---|
| External Links |
|---|
| DrugBank ID | Not Available |
|---|
| HMDB ID | HMDB0032193 |
|---|
| FooDB ID | FDB009066 |
|---|
| Phenol Explorer ID | Not Available |
|---|
| KNApSAcK ID | Not Available |
|---|
| BiGG ID | Not Available |
|---|
| BioCyc ID | Not Available |
|---|
| METLIN ID | Not Available |
|---|
| PDB ID | Not Available |
|---|
| Wikipedia Link | Not Available |
|---|
| Chemspider ID | 55209 |
|---|
| ChEBI ID | 172065 |
|---|
| PubChem Compound ID | 61270 |
|---|
| Kegg Compound ID | Not Available |
|---|
| YMDB ID | Not Available |
|---|
| ECMDB ID | Not Available |
|---|
| References |
|---|
| Synthesis Reference | Not Available |
|---|
| MSDS | Not Available |
|---|
| General References | |
|---|