| Record Information |
|---|
| Version | 1.0 |
|---|
| Creation Date | 2016-05-19 01:56:00 UTC |
|---|
| Update Date | 2016-11-09 01:09:26 UTC |
|---|
| Accession Number | CHEM004889 |
|---|
| Identification |
|---|
| Common Name | BUTTER ACIDS |
|---|
| Class | Small Molecule |
|---|
| Description | Butter acids is found in milk and milk products. Butter acids is a flavouring ingredient for baked goods, ice cream, puddings, imitation dairy products, nonalcoholic beverages and candies. |
|---|
| Contaminant Sources | - EAFUS Chemicals
- FooDB Chemicals
|
|---|
| Contaminant Type | Not Available |
|---|
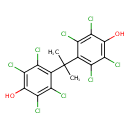
| Chemical Structure | |
|---|
| Synonyms | | Value | Source |
|---|
| 4,4'-(1-Methylethylidene)bis(2,3,5,6-tetrachloro-phenol | HMDB | | FEMA 2171 | HMDB | | Isopropylidene bis-tetrachlorophenol | HMDB |
|
|---|
| Chemical Formula | C15H8Cl8O2 |
|---|
| Average Molecular Mass | 503.847 g/mol |
|---|
| Monoisotopic Mass | 499.803 g/mol |
|---|
| CAS Registry Number | 85536-25-0 |
|---|
| IUPAC Name | 2,3,5,6-tetrachloro-4-[2-(2,3,5,6-tetrachloro-4-hydroxyphenyl)propan-2-yl]phenol |
|---|
| Traditional Name | 2,3,5,6-tetrachloro-4-[2-(2,3,5,6-tetrachloro-4-hydroxyphenyl)propan-2-yl]phenol |
|---|
| SMILES | CC(C)(C1=C(Cl)C(Cl)=C(O)C(Cl)=C1Cl)C1=C(Cl)C(Cl)=C(O)C(Cl)=C1Cl |
|---|
| InChI Identifier | InChI=1S/C15H8Cl8O2/c1-15(2,3-5(16)9(20)13(24)10(21)6(3)17)4-7(18)11(22)14(25)12(23)8(4)19/h24-25H,1-2H3 |
|---|
| InChI Key | OJTHLNYBRBMCBW-UHFFFAOYSA-N |
|---|
| Chemical Taxonomy |
|---|
| Description | belongs to the class of organic compounds known as bisphenols. These are methylenediphenols, HOC6H4CH2C6H4OH, commonly p,p-methylenediphenol, and their substitution products (generally derived from condensation of two equivalent amounts of a phenol with an aldehyde or ketone). |
|---|
| Kingdom | Organic compounds |
|---|
| Super Class | Benzenoids |
|---|
| Class | Benzene and substituted derivatives |
|---|
| Sub Class | Diphenylmethanes |
|---|
| Direct Parent | Bisphenols |
|---|
| Alternative Parents | |
|---|
| Substituents | - Bisphenol
- Phenylpropane
- 3-halophenol
- 2-halophenol
- 3-chlorophenol
- 2-chlorophenol
- Chlorobenzene
- Halobenzene
- Phenol
- Aryl halide
- Aryl chloride
- Hydrocarbon derivative
- Organic oxygen compound
- Organooxygen compound
- Organochloride
- Organohalogen compound
- Aromatic homomonocyclic compound
|
|---|
| Molecular Framework | Aromatic homomonocyclic compounds |
|---|
| External Descriptors | Not Available |
|---|
| Biological Properties |
|---|
| Status | Detected and Not Quantified |
|---|
| Origin | Not Available |
|---|
| Cellular Locations | Not Available |
|---|
| Biofluid Locations | Not Available |
|---|
| Tissue Locations | Not Available |
|---|
| Pathways | Not Available |
|---|
| Applications | Not Available |
|---|
| Biological Roles | Not Available |
|---|
| Chemical Roles | Not Available |
|---|
| Physical Properties |
|---|
| State | Not Available |
|---|
| Appearance | Not Available |
|---|
| Experimental Properties | | Property | Value |
|---|
| Melting Point | Not Available | | Boiling Point | Not Available | | Solubility | Not Available |
|
|---|
| Predicted Properties | |
|---|
| Spectra |
|---|
| Spectra | | Spectrum Type | Description | Splash Key | View |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | splash10-0fe3-2050920000-1b75fd351f8f28b371f2 | Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (2 TMS) - 70eV, Positive | splash10-00e9-9003005000-5ab2e695c11a764fabb1 | Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | Not Available | Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | Not Available | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-0udi-0000090000-45aaf561c0b6af285064 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-0udi-0000090000-aece6d705423b0099e71 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-0ul0-0090210000-25c4ecc993b3727542e5 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-0002-0020900000-3f4e7afaf8310feebb9d | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-0002-0000900000-92009bdc278eb69e1c70 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-004j-0290800000-1e817175a0288b28efa0 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-0002-0000900000-405ddc6757ca18006f66 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-0002-0000900000-405ddc6757ca18006f66 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-000t-0020900000-8fce34092fa573271aee | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-0udi-0000090000-61c9339e285fa544aae4 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-0udi-0000090000-61c9339e285fa544aae4 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-0una-0004930000-142cfbcc3d8a17b50561 | Spectrum |
|
|---|
| Toxicity Profile |
|---|
| Route of Exposure | Not Available |
|---|
| Mechanism of Toxicity | Not Available |
|---|
| Metabolism | Not Available |
|---|
| Toxicity Values | Not Available |
|---|
| Lethal Dose | Not Available |
|---|
| Carcinogenicity (IARC Classification) | Not Available |
|---|
| Uses/Sources | Not Available |
|---|
| Minimum Risk Level | Not Available |
|---|
| Health Effects | Not Available |
|---|
| Symptoms | Not Available |
|---|
| Treatment | Not Available |
|---|
| Concentrations |
|---|
| Not Available |
|---|
| External Links |
|---|
| DrugBank ID | Not Available |
|---|
| HMDB ID | HMDB0039842 |
|---|
| FooDB ID | FDB019496 |
|---|
| Phenol Explorer ID | Not Available |
|---|
| KNApSAcK ID | Not Available |
|---|
| BiGG ID | Not Available |
|---|
| BioCyc ID | Not Available |
|---|
| METLIN ID | Not Available |
|---|
| PDB ID | Not Available |
|---|
| Wikipedia Link | Not Available |
|---|
| Chemspider ID | 77143 |
|---|
| ChEBI ID | Not Available |
|---|
| PubChem Compound ID | 85536 |
|---|
| Kegg Compound ID | Not Available |
|---|
| YMDB ID | Not Available |
|---|
| ECMDB ID | Not Available |
|---|
| References |
|---|
| Synthesis Reference | Not Available |
|---|
| MSDS | Not Available |
|---|
| General References | |
|---|