| Record Information |
|---|
| Version | 1.0 |
|---|
| Creation Date | 2016-05-19 01:55:56 UTC |
|---|
| Update Date | 2016-11-09 01:09:26 UTC |
|---|
| Accession Number | CHEM004884 |
|---|
| Identification |
|---|
| Common Name | 1-BUTANETHIOL |
|---|
| Class | Small Molecule |
|---|
| Description | 1-Butanethiol is found in animal foods. 1-Butanethiol is a flavouring agent. 1-Butanethiol is present in beef, Cheshire cheese, raw chicken and cooked potatoe |
|---|
| Contaminant Sources | - EAFUS Chemicals
- FooDB Chemicals
- HPV EPA Chemicals
- OECD HPV Chemicals
- ToxCast & Tox21 Chemicals
|
|---|
| Contaminant Type | Not Available |
|---|
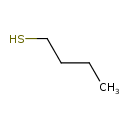
| Chemical Structure | |
|---|
| Synonyms | | Value | Source |
|---|
| 1-Butyl mercaptan | HMDB | | 1-Butylthiol | HMDB | | 1-Mercaptobutane | HMDB | | Bear skunk | HMDB | | Butane-1-thiol | HMDB | | Butanethiol | HMDB, MeSH | | Butyl mercaptan | HMDB | | Butyl thioalcohol | HMDB | | Butylthiol | HMDB | | FEMA 3478 | HMDB | | Mercaptan C4 | HMDB | | N-Butanethiol | HMDB | | N-Butyl mercaptan | HMDB, MeSH | | N-Butyl mercaptan, 1,2-(14)C,2-(35) S-labeled CPD | HMDB, MeSH | | N-Butyl mercaptan, 14C,1-(35)S-labeled CPD | HMDB, MeSH | | N-Butyl mercaptan, 2-(14)C,2-(35)S-labeled CPD | HMDB, MeSH | | N-Butyl mercaptan, ag(+1) salt | HMDB, MeSH | | N-Butyl mercaptan, copper (+1) salt | HMDB, MeSH | | N-Butyl mercaptan, geranium (+2) salt | HMDB, MeSH | | N-Butyl mercaptan, lead (+2) salt | HMDB, MeSH | | N-Butyl mercaptan, lithium salt | HMDB, MeSH | | N-Butyl mercaptan, molybdenum (+3) salt | HMDB, MeSH | | N-Butyl mercaptan, potassium salt | HMDB, MeSH | | N-Butyl mercaptan, silver (+2) salt | HMDB, MeSH | | N-Butyl mercaptan, sodium salt | HMDB, MeSH | | N-Butyl mercaptan, tin (+2) salt | HMDB, MeSH | | N-Butyl thioalcohol | HMDB | | N-Butylmercaptan | HMDB | | N-Butylthiol | HMDB | | N-C4H9SH | HMDB | | Normal butyl thioalcohol | HMDB | | Thiobutyl alcohol | HMDB |
|
|---|
| Chemical Formula | C4H10S |
|---|
| Average Molecular Mass | 90.187 g/mol |
|---|
| Monoisotopic Mass | 90.050 g/mol |
|---|
| CAS Registry Number | 109-79-5 |
|---|
| IUPAC Name | butane-1-thiol |
|---|
| Traditional Name | butanethiol |
|---|
| SMILES | CCCCS |
|---|
| InChI Identifier | InChI=1S/C4H10S/c1-2-3-4-5/h5H,2-4H2,1H3 |
|---|
| InChI Key | WQAQPCDUOCURKW-UHFFFAOYSA-N |
|---|
| Chemical Taxonomy |
|---|
| Description | belongs to the class of organic compounds known as alkylthiols. These are organic compounds containing the thiol functional group linked to an alkyl chain. |
|---|
| Kingdom | Organic compounds |
|---|
| Super Class | Organosulfur compounds |
|---|
| Class | Thiols |
|---|
| Sub Class | Alkylthiols |
|---|
| Direct Parent | Alkylthiols |
|---|
| Alternative Parents | |
|---|
| Substituents | - Alkylthiol
- Hydrocarbon derivative
- Aliphatic acyclic compound
|
|---|
| Molecular Framework | Aliphatic acyclic compounds |
|---|
| External Descriptors | Not Available |
|---|
| Biological Properties |
|---|
| Status | Detected and Not Quantified |
|---|
| Origin | Not Available |
|---|
| Cellular Locations | Not Available |
|---|
| Biofluid Locations | Not Available |
|---|
| Tissue Locations | Not Available |
|---|
| Pathways | Not Available |
|---|
| Applications | Not Available |
|---|
| Biological Roles | Not Available |
|---|
| Chemical Roles | Not Available |
|---|
| Physical Properties |
|---|
| State | Not Available |
|---|
| Appearance | Not Available |
|---|
| Experimental Properties | | Property | Value |
|---|
| Melting Point | Not Available | | Boiling Point | Not Available | | Solubility | Not Available |
|
|---|
| Predicted Properties | |
|---|
| Spectra |
|---|
| Spectra | | Spectrum Type | Description | Splash Key | View |
|---|
| GC-MS | GC-MS Spectrum - EI-B (Non-derivatized) | splash10-054o-9000000000-a6dc170a091918ad8ebd | Spectrum | | GC-MS | GC-MS Spectrum - EI-B (Non-derivatized) | splash10-054o-9000000000-a6dc170a091918ad8ebd | Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | splash10-056u-9000000000-38925ee4986be533057a | Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | Not Available | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-0006-9000000000-2c8931195c68fecc8111 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-052f-9000000000-8dd6c489c186d5933822 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-0a4l-9000000000-80366c7db7d0b5b47c95 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-000i-9000000000-d17a59485a19101bb39a | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-000i-9000000000-74dda8912841aec47b87 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-001i-9000000000-531ef776f2658efbb595 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-000i-9000000000-c15245c3ae5818f2f713 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-000i-9000000000-b0336cfaef3fe02ff577 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-001i-9000000000-942ac689538269d6ca7b | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-052f-9000000000-5674c9f66c1431240aae | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-0a4i-9000000000-9dad4faebaa201504e70 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-0005-9000000000-a78a12fe80d1c82aa4d4 | Spectrum | | MS | Mass Spectrum (Electron Ionization) | splash10-054o-9000000000-82c51a7b87bcecf17695 | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum |
|
|---|
| Toxicity Profile |
|---|
| Route of Exposure | Not Available |
|---|
| Mechanism of Toxicity | Not Available |
|---|
| Metabolism | Not Available |
|---|
| Toxicity Values | Not Available |
|---|
| Lethal Dose | Not Available |
|---|
| Carcinogenicity (IARC Classification) | Not Available |
|---|
| Uses/Sources | Not Available |
|---|
| Minimum Risk Level | Not Available |
|---|
| Health Effects | Not Available |
|---|
| Symptoms | Not Available |
|---|
| Treatment | Not Available |
|---|
| Concentrations |
|---|
| Not Available |
|---|
| External Links |
|---|
| DrugBank ID | Not Available |
|---|
| HMDB ID | HMDB0031322 |
|---|
| FooDB ID | FDB003381 |
|---|
| Phenol Explorer ID | Not Available |
|---|
| KNApSAcK ID | C00050484 |
|---|
| BiGG ID | Not Available |
|---|
| BioCyc ID | Not Available |
|---|
| METLIN ID | Not Available |
|---|
| PDB ID | Not Available |
|---|
| Wikipedia Link | Butanethiol |
|---|
| Chemspider ID | 7721 |
|---|
| ChEBI ID | Not Available |
|---|
| PubChem Compound ID | 8012 |
|---|
| Kegg Compound ID | Not Available |
|---|
| YMDB ID | Not Available |
|---|
| ECMDB ID | Not Available |
|---|
| References |
|---|
| Synthesis Reference | Not Available |
|---|
| MSDS | Not Available |
|---|
| General References | |
|---|