| Record Information |
|---|
| Version | 1.0 |
|---|
| Creation Date | 2016-05-19 01:55:48 UTC |
|---|
| Update Date | 2016-11-09 01:09:26 UTC |
|---|
| Accession Number | CHEM004870 |
|---|
| Identification |
|---|
| Common Name | BROMELAIN |
|---|
| Class | Small Molecule |
|---|
| Description | Not Available |
|---|
| Contaminant Sources | - EAFUS Chemicals
- FooDB Chemicals
|
|---|
| Contaminant Type | Not Available |
|---|
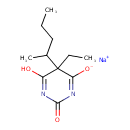
| Chemical Structure | |
|---|
| Synonyms | | Value | Source |
|---|
| Nembutal sodium | Kegg | | Sodium 5-ethyl-6-hydroxy-2-oxo-5-(pentan-2-yl)-2,5-dihydropyrimidin-4-olic acid | Generator | | Monosodium salt pentobarbital | MeSH | | Pentobarbital | MeSH | | Pentobarbitone | MeSH | | Sagatal | MeSH | | Diabutal | MeSH | | Mebubarbital | MeSH | | Nembutal | MeSH | | Mebumal | MeSH | | Etaminal | MeSH | | Ethaminal | MeSH | | Pentobarbital, monosodium salt | MeSH |
|
|---|
| Chemical Formula | C11H17N2NaO3 |
|---|
| Average Molecular Mass | 248.254 g/mol |
|---|
| Monoisotopic Mass | 248.114 g/mol |
|---|
| CAS Registry Number | 9001-00-7 |
|---|
| IUPAC Name | sodium 5-ethyl-6-hydroxy-2-oxo-5-(pentan-2-yl)-2,5-dihydropyrimidin-4-olate |
|---|
| Traditional Name | sodium 5-ethyl-6-hydroxy-2-oxo-5-(pentan-2-yl)pyrimidin-4-olate |
|---|
| SMILES | [Na+].CCCC(C)C1(CC)C(O)=NC(=O)N=C1[O-] |
|---|
| InChI Identifier | InChI=1S/C11H18N2O3.Na/c1-4-6-7(3)11(5-2)8(14)12-10(16)13-9(11)15;/h7H,4-6H2,1-3H3,(H2,12,13,14,15,16);/q;+1/p-1 |
|---|
| InChI Key | QGMRQYFBGABWDR-UHFFFAOYSA-M |
|---|
| Chemical Taxonomy |
|---|
| Description | belongs to the class of organic compounds known as barbituric acid derivatives. Barbituric acid derivatives are compounds containing a perhydropyrimidine ring substituted at C-2, -4 and -6 by oxo groups. |
|---|
| Kingdom | Organic compounds |
|---|
| Super Class | Organoheterocyclic compounds |
|---|
| Class | Diazines |
|---|
| Sub Class | Pyrimidines and pyrimidine derivatives |
|---|
| Direct Parent | Barbituric acid derivatives |
|---|
| Alternative Parents | |
|---|
| Substituents | - Barbiturate
- N-acyl urea
- Ureide
- 1,3-diazinane
- Dicarboximide
- Carbonic acid derivative
- Carboxylic acid derivative
- Azacycle
- Carbene-type 1,3-dipolar compound
- Organic 1,3-dipolar compound
- Organic alkali metal salt
- Organic nitrogen compound
- Organooxygen compound
- Organonitrogen compound
- Hydrocarbon derivative
- Organic oxide
- Organopnictogen compound
- Carbonyl group
- Organic salt
- Organic sodium salt
- Organic oxygen compound
- Aliphatic heteromonocyclic compound
|
|---|
| Molecular Framework | Aliphatic heteromonocyclic compounds |
|---|
| External Descriptors | |
|---|
| Biological Properties |
|---|
| Status | Detected and Not Quantified |
|---|
| Origin | Not Available |
|---|
| Cellular Locations | Not Available |
|---|
| Biofluid Locations | Not Available |
|---|
| Tissue Locations | Not Available |
|---|
| Pathways | Not Available |
|---|
| Applications | Not Available |
|---|
| Biological Roles | Not Available |
|---|
| Chemical Roles | Not Available |
|---|
| Physical Properties |
|---|
| State | Not Available |
|---|
| Appearance | Not Available |
|---|
| Experimental Properties | | Property | Value |
|---|
| Melting Point | Not Available | | Boiling Point | Not Available | | Solubility | Not Available |
|
|---|
| Predicted Properties | |
|---|
| Spectra |
|---|
| Spectra | | Spectrum Type | Description | Splash Key | View |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-0002-7190000000-eec15e20d0a156e71f22 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-052g-9770000000-bd1aa3d56755cce16058 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-0006-9000000000-27b3b12bd8afcfd82ded | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-000w-3590000000-c300861382ca87b5ed29 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-0006-9300000000-5e32d57457c911eb7d06 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-0006-9700000000-5a466fde2779164bb06a | Spectrum |
|
|---|
| Toxicity Profile |
|---|
| Route of Exposure | Not Available |
|---|
| Mechanism of Toxicity | Not Available |
|---|
| Metabolism | Not Available |
|---|
| Toxicity Values | Not Available |
|---|
| Lethal Dose | Not Available |
|---|
| Carcinogenicity (IARC Classification) | Not Available |
|---|
| Uses/Sources | Not Available |
|---|
| Minimum Risk Level | Not Available |
|---|
| Health Effects | Not Available |
|---|
| Symptoms | Not Available |
|---|
| Treatment | Not Available |
|---|
| Concentrations |
|---|
| Not Available |
|---|
| External Links |
|---|
| DrugBank ID | DBSALT000442 |
|---|
| HMDB ID | HMDB0304826 |
|---|
| FooDB ID | FDB001083 |
|---|
| Phenol Explorer ID | Not Available |
|---|
| KNApSAcK ID | Not Available |
|---|
| BiGG ID | Not Available |
|---|
| BioCyc ID | Not Available |
|---|
| METLIN ID | Not Available |
|---|
| PDB ID | Not Available |
|---|
| Wikipedia Link | Not Available |
|---|
| Chemspider ID | 5762 |
|---|
| ChEBI ID | Not Available |
|---|
| PubChem Compound ID | 5982 |
|---|
| Kegg Compound ID | C07423 |
|---|
| YMDB ID | Not Available |
|---|
| ECMDB ID | Not Available |
|---|
| References |
|---|
| Synthesis Reference | Not Available |
|---|
| MSDS | Not Available |
|---|
| General References | Not Available |
|---|