| Record Information |
|---|
| Version | 1.0 |
|---|
| Creation Date | 2016-05-19 01:55:35 UTC |
|---|
| Update Date | 2016-11-09 01:09:26 UTC |
|---|
| Accession Number | CHEM004849 |
|---|
| Identification |
|---|
| Common Name | BIS(2,5-DIMETHYL-3-FURYL) DISULFIDE |
|---|
| Class | Small Molecule |
|---|
| Description | Bis(2,5-dimethyl-3-furanyl) disulfide is a synthetic meat flavouring agent |
|---|
| Contaminant Sources | - EAFUS Chemicals
- FooDB Chemicals
|
|---|
| Contaminant Type | Not Available |
|---|
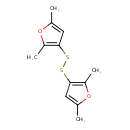
| Chemical Structure | |
|---|
| Synonyms | | Value | Source |
|---|
| Bis(2,5-dimethyl-3-furanyl) disulphide | Generator | | 3,3'-Dithiobis(2,5-dimethyl-furan | HMDB | | 3,3'-Dithiobis(2,5-dimethylfuran) | HMDB | | 3,3'-Dithiobis[2,5-dimethyl-furan | HMDB | | 3,3'-Dithiobis[2,5-dimethylfuran], 9ci, 8ci | HMDB | | Bis(2,5-dimethyl-3-furyl) disulfide | HMDB | | FEMA 3476 | HMDB | | 3-[(2,5-Dimethylfuran-3-yl)disulphanyl]-2,5-dimethylfuran | Generator |
|
|---|
| Chemical Formula | C12H14O2S2 |
|---|
| Average Molecular Mass | 254.368 g/mol |
|---|
| Monoisotopic Mass | 254.044 g/mol |
|---|
| CAS Registry Number | 28588-73-0 |
|---|
| IUPAC Name | 3-[(2,5-dimethylfuran-3-yl)disulfanyl]-2,5-dimethylfuran |
|---|
| Traditional Name | 3-[(2,5-dimethylfuran-3-yl)disulfanyl]-2,5-dimethylfuran |
|---|
| SMILES | CC1=CC(SSC2=C(C)OC(C)=C2)=C(C)O1 |
|---|
| InChI Identifier | InChI=1S/C12H14O2S2/c1-7-5-11(9(3)13-7)15-16-12-6-8(2)14-10(12)4/h5-6H,1-4H3 |
|---|
| InChI Key | JDWCALSZHJBMIQ-UHFFFAOYSA-N |
|---|
| Chemical Taxonomy |
|---|
| Description | belongs to the class of organic compounds known as heteroaromatic compounds. Heteroaromatic compounds are compounds containing an aromatic ring where a carbon atom is linked to an hetero atom. |
|---|
| Kingdom | Organic compounds |
|---|
| Super Class | Organoheterocyclic compounds |
|---|
| Class | Heteroaromatic compounds |
|---|
| Sub Class | Not Available |
|---|
| Direct Parent | Heteroaromatic compounds |
|---|
| Alternative Parents | |
|---|
| Substituents | - Heteroaromatic compound
- Furan
- Organic disulfide
- Oxacycle
- Sulfenyl compound
- Organic oxygen compound
- Hydrocarbon derivative
- Organosulfur compound
- Organooxygen compound
- Aromatic heteromonocyclic compound
|
|---|
| Molecular Framework | Aromatic heteromonocyclic compounds |
|---|
| External Descriptors | Not Available |
|---|
| Biological Properties |
|---|
| Status | Detected and Not Quantified |
|---|
| Origin | Not Available |
|---|
| Cellular Locations | Not Available |
|---|
| Biofluid Locations | Not Available |
|---|
| Tissue Locations | Not Available |
|---|
| Pathways | Not Available |
|---|
| Applications | Not Available |
|---|
| Biological Roles | Not Available |
|---|
| Chemical Roles | Not Available |
|---|
| Physical Properties |
|---|
| State | Not Available |
|---|
| Appearance | Not Available |
|---|
| Experimental Properties | | Property | Value |
|---|
| Melting Point | Not Available | | Boiling Point | Not Available | | Solubility | Not Available |
|
|---|
| Predicted Properties | |
|---|
| Spectra |
|---|
| Spectra | | Spectrum Type | Description | Splash Key | View |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | splash10-004i-8950000000-69a2fc73059db9ba5b86 | Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | Not Available | Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | Not Available | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-0a4i-3290000000-1691f762f2e7525f2248 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-08fr-4590000000-317be92494bc0d48d650 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-000f-9200000000-edecec0990599f3e7bb3 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-0udi-0290000000-17bce2bcfc78b0e72de6 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-004i-4920000000-63805c16bf5684ff5c8d | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-066v-9620000000-f10a90e9ea714c456166 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-004i-1910000000-e20d341e97264e86f602 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-004i-6900000000-487aae7a4cf5c593915e | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-005d-9410000000-a049a49e9b35230de2a2 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-004i-0920000000-cbd8819f5315b1321f90 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-0zj1-8900000000-589d50fc5269617528d4 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-08fv-5900000000-63de8b5b58b2503dafaa | Spectrum |
|
|---|
| Toxicity Profile |
|---|
| Route of Exposure | Not Available |
|---|
| Mechanism of Toxicity | Not Available |
|---|
| Metabolism | Not Available |
|---|
| Toxicity Values | Not Available |
|---|
| Lethal Dose | Not Available |
|---|
| Carcinogenicity (IARC Classification) | Not Available |
|---|
| Uses/Sources | Not Available |
|---|
| Minimum Risk Level | Not Available |
|---|
| Health Effects | Not Available |
|---|
| Symptoms | Not Available |
|---|
| Treatment | Not Available |
|---|
| Concentrations |
|---|
| Not Available |
|---|
| External Links |
|---|
| DrugBank ID | Not Available |
|---|
| HMDB ID | HMDB0036167 |
|---|
| FooDB ID | FDB015021 |
|---|
| Phenol Explorer ID | Not Available |
|---|
| KNApSAcK ID | Not Available |
|---|
| BiGG ID | Not Available |
|---|
| BioCyc ID | Not Available |
|---|
| METLIN ID | Not Available |
|---|
| PDB ID | Not Available |
|---|
| Wikipedia Link | Not Available |
|---|
| Chemspider ID | 557342 |
|---|
| ChEBI ID | Not Available |
|---|
| PubChem Compound ID | 642117 |
|---|
| Kegg Compound ID | Not Available |
|---|
| YMDB ID | Not Available |
|---|
| ECMDB ID | Not Available |
|---|
| References |
|---|
| Synthesis Reference | Not Available |
|---|
| MSDS | Not Available |
|---|
| General References | |
|---|