| Record Information |
|---|
| Version | 1.0 |
|---|
| Creation Date | 2016-05-19 01:55:20 UTC |
|---|
| Update Date | 2016-11-09 01:09:25 UTC |
|---|
| Accession Number | CHEM004826 |
|---|
| Identification |
|---|
| Common Name | BENZYL 2,3-DIMETHYLCROTONATE |
|---|
| Class | Small Molecule |
|---|
| Description | Benzyl 2,3-dimethyl-2-butenoate is a flavouring agent |
|---|
| Contaminant Sources | - EAFUS Chemicals
- FooDB Chemicals
|
|---|
| Contaminant Type | Not Available |
|---|
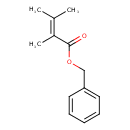
| Chemical Structure | |
|---|
| Synonyms | | Value | Source |
|---|
| Benzyl 2,3-dimethyl-2-butenoic acid | Generator | | 2-Butenoic acid, 2,3-dimethyl-, phenylmethyl ester | HMDB | | Benzyl 2,3-dimethylcrotonate | HMDB | | Benzyl methyltiglate | HMDB | | Crotonic acid, 2,3-dimethyl-, benzyl ester | HMDB | | FEMA 2143 | HMDB | | Phenylmethyl 2,3-dimethyl-2-butenoate | HMDB | | Benzyl 2,3-dimethylbut-2-enoic acid | Generator |
|
|---|
| Chemical Formula | C13H16O2 |
|---|
| Average Molecular Mass | 204.265 g/mol |
|---|
| Monoisotopic Mass | 204.115 g/mol |
|---|
| CAS Registry Number | 7492-69-5 |
|---|
| IUPAC Name | benzyl 2,3-dimethylbut-2-enoate |
|---|
| Traditional Name | benzyl 2,3-dimethylbut-2-enoate |
|---|
| SMILES | CC(C)=C(C)C(=O)OCC1=CC=CC=C1 |
|---|
| InChI Identifier | InChI=1S/C13H16O2/c1-10(2)11(3)13(14)15-9-12-7-5-4-6-8-12/h4-8H,9H2,1-3H3 |
|---|
| InChI Key | LHDWSNQMWAZQPX-UHFFFAOYSA-N |
|---|
| Chemical Taxonomy |
|---|
| Description | belongs to the class of organic compounds known as benzyloxycarbonyls. These are organic compounds containing a carbonyl group substituted with a benzyloxyl group. |
|---|
| Kingdom | Organic compounds |
|---|
| Super Class | Benzenoids |
|---|
| Class | Benzene and substituted derivatives |
|---|
| Sub Class | Benzyloxycarbonyls |
|---|
| Direct Parent | Benzyloxycarbonyls |
|---|
| Alternative Parents | |
|---|
| Substituents | - Benzyloxycarbonyl
- Fatty acid ester
- Fatty acyl
- Alpha,beta-unsaturated carboxylic ester
- Enoate ester
- Carboxylic acid ester
- Monocarboxylic acid or derivatives
- Carboxylic acid derivative
- Organic oxygen compound
- Organic oxide
- Hydrocarbon derivative
- Organooxygen compound
- Carbonyl group
- Aromatic homomonocyclic compound
|
|---|
| Molecular Framework | Aromatic homomonocyclic compounds |
|---|
| External Descriptors | Not Available |
|---|
| Biological Properties |
|---|
| Status | Detected and Not Quantified |
|---|
| Origin | Not Available |
|---|
| Cellular Locations | Not Available |
|---|
| Biofluid Locations | Not Available |
|---|
| Tissue Locations | Not Available |
|---|
| Pathways | Not Available |
|---|
| Applications | Not Available |
|---|
| Biological Roles | Not Available |
|---|
| Chemical Roles | Not Available |
|---|
| Physical Properties |
|---|
| State | Not Available |
|---|
| Appearance | Not Available |
|---|
| Experimental Properties | | Property | Value |
|---|
| Melting Point | Not Available | | Boiling Point | Not Available | | Solubility | Not Available |
|
|---|
| Predicted Properties | |
|---|
| Spectra |
|---|
| Spectra | | Spectrum Type | Description | Splash Key | View |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | splash10-0006-9000000000-455a6ceef170239723b4 | Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | Not Available | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-0a4l-9160000000-b503940751dace6e8a3b | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-0006-9000000000-5d645eb437a6a30709e9 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-0006-9000000000-576eba00a3b712ac9815 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-0udi-2190000000-dc2026ec5d09ea6ae15c | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-0j4j-9530000000-54cbba632e07d9e4b4b3 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-016r-9100000000-809f8e85d5bc286d8946 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-014i-9000000000-85dffab9cc12c05a9564 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-00kf-9000000000-7c3db47bd1d6eea59205 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-0006-9000000000-b332bc58d9ec6fc6f189 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-0udi-0190000000-5bbcf3349c9ba43b9d97 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-0006-9300000000-98ea6f602661f7ac5511 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-016r-9100000000-bccdccd5451fad9226cf | Spectrum |
|
|---|
| Toxicity Profile |
|---|
| Route of Exposure | Not Available |
|---|
| Mechanism of Toxicity | Not Available |
|---|
| Metabolism | Not Available |
|---|
| Toxicity Values | Not Available |
|---|
| Lethal Dose | Not Available |
|---|
| Carcinogenicity (IARC Classification) | Not Available |
|---|
| Uses/Sources | Not Available |
|---|
| Minimum Risk Level | Not Available |
|---|
| Health Effects | Not Available |
|---|
| Symptoms | Not Available |
|---|
| Treatment | Not Available |
|---|
| Concentrations |
|---|
| Not Available |
|---|
| External Links |
|---|
| DrugBank ID | Not Available |
|---|
| HMDB ID | HMDB0036224 |
|---|
| FooDB ID | FDB015082 |
|---|
| Phenol Explorer ID | Not Available |
|---|
| KNApSAcK ID | Not Available |
|---|
| BiGG ID | Not Available |
|---|
| BioCyc ID | Not Available |
|---|
| METLIN ID | Not Available |
|---|
| PDB ID | Not Available |
|---|
| Wikipedia Link | Not Available |
|---|
| Chemspider ID | 55331 |
|---|
| ChEBI ID | Not Available |
|---|
| PubChem Compound ID | 61402 |
|---|
| Kegg Compound ID | Not Available |
|---|
| YMDB ID | Not Available |
|---|
| ECMDB ID | Not Available |
|---|
| References |
|---|
| Synthesis Reference | Not Available |
|---|
| MSDS | Not Available |
|---|
| General References | |
|---|