| Record Information |
|---|
| Version | 1.0 |
|---|
| Creation Date | 2016-05-19 01:54:50 UTC |
|---|
| Update Date | 2016-11-09 01:09:25 UTC |
|---|
| Accession Number | CHEM004788 |
|---|
| Identification |
|---|
| Common Name | BAKERS YEAST EXTRACT |
|---|
| Class | Small Molecule |
|---|
| Description | Bakers yeast extract is used as a food additive [EAFUS] ("EAFUS: Everything Added to Food in the United States. [http://www.eafus.com/]") |
|---|
| Contaminant Sources | - EAFUS Chemicals
- FooDB Chemicals
|
|---|
| Contaminant Type | Not Available |
|---|
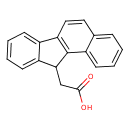
| Chemical Structure | |
|---|
| Synonyms | | Value | Source |
|---|
| (11H-Benzo[a]fluoren-11-yl)acetic acid | ChEBI | | 2-(11H-Benzo[a]luoren-11-yl)acetic acid | ChEBI | | (11H-Benzo[a]fluoren-11-yl)acetate | Generator | | 2-(11H-Benzo[a]luoren-11-yl)acetate | Generator | | 11H-Benzo[a]fluoren-11-ylacetic acid | HMDB | | 2-{tetracyclo[8.7.0.0²,⁷.0¹¹,¹⁶]heptadeca-1(10),2,4,6,8,11,13,15-octaen-17-yl}acetate | HMDB |
|
|---|
| Chemical Formula | C19H14O2 |
|---|
| Average Molecular Mass | 274.313 g/mol |
|---|
| Monoisotopic Mass | 274.099 g/mol |
|---|
| CAS Registry Number | 8013-01-2 |
|---|
| IUPAC Name | 2-{tetracyclo[8.7.0.0²,⁷.0¹¹,¹⁶]heptadeca-1(10),2,4,6,8,11,13,15-octaen-17-yl}acetic acid |
|---|
| Traditional Name | tetracyclo[8.7.0.0²,⁷.0¹¹,¹⁶]heptadeca-1(10),2,4,6,8,11,13,15-octaen-17-ylacetic acid |
|---|
| SMILES | OC(=O)CC1C2=CC=CC=C2C2=C1C1=CC=CC=C1C=C2 |
|---|
| InChI Identifier | InChI=1S/C19H14O2/c20-18(21)11-17-15-8-4-3-7-14(15)16-10-9-12-5-1-2-6-13(12)19(16)17/h1-10,17H,11H2,(H,20,21) |
|---|
| InChI Key | GQNBDGXKDJSVGQ-UHFFFAOYSA-N |
|---|
| Chemical Taxonomy |
|---|
| Description | belongs to the class of organic compounds known as fluorenes. Fluorenes are compounds containing a fluorene moiety, which consists of two benzene rings connected through either a cyclopentane, cyclopentene, or cyclopenta-1,3-diene. |
|---|
| Kingdom | Organic compounds |
|---|
| Super Class | Benzenoids |
|---|
| Class | Fluorenes |
|---|
| Sub Class | Not Available |
|---|
| Direct Parent | Fluorenes |
|---|
| Alternative Parents | |
|---|
| Substituents | - Fluorene
- Naphthalene
- Monocarboxylic acid or derivatives
- Carboxylic acid
- Carboxylic acid derivative
- Organic oxygen compound
- Organic oxide
- Hydrocarbon derivative
- Organooxygen compound
- Carbonyl group
- Aromatic homopolycyclic compound
|
|---|
| Molecular Framework | Aromatic homopolycyclic compounds |
|---|
| External Descriptors | Not Available |
|---|
| Biological Properties |
|---|
| Status | Detected and Not Quantified |
|---|
| Origin | Not Available |
|---|
| Cellular Locations | Not Available |
|---|
| Biofluid Locations | Not Available |
|---|
| Tissue Locations | Not Available |
|---|
| Pathways | Not Available |
|---|
| Applications | Not Available |
|---|
| Biological Roles | Not Available |
|---|
| Chemical Roles | Not Available |
|---|
| Physical Properties |
|---|
| State | Not Available |
|---|
| Appearance | Not Available |
|---|
| Experimental Properties | | Property | Value |
|---|
| Melting Point | Not Available | | Boiling Point | Not Available | | Solubility | Not Available |
|
|---|
| Predicted Properties | |
|---|
| Spectra |
|---|
| Spectra | | Spectrum Type | Description | Splash Key | View |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | splash10-004j-0190000000-823eb1f0bfbc44b14869 | Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (1 TMS) - 70eV, Positive | splash10-00c0-9163000000-f8752de81b5fef113973 | Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | Not Available | Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | Not Available | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-056r-0090000000-cb0031e5095cdaeae48a | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-004i-0090000000-7f0a3847e8add7502219 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-004i-2290000000-68e8d326747dff93c7ae | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-00fr-0090000000-a1167ed0ea9f180772bb | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-00b9-0090000000-a330ddcca315adcdceb2 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-06vl-3090000000-4f714aaa67edf385b235 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-004i-0090000000-539cf3a4bc9dc97126d0 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-004i-0090000000-94f5bcc8af54bd748ea2 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-004i-0090000000-05e44da82e889aeebdf4 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-00di-0090000000-859b0df98a228d675d41 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-004i-0090000000-19ab1e47b2c7ec112b5e | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-004i-0090000000-561e3cf0020c0fd34428 | Spectrum |
|
|---|
| Toxicity Profile |
|---|
| Route of Exposure | Not Available |
|---|
| Mechanism of Toxicity | Not Available |
|---|
| Metabolism | Not Available |
|---|
| Toxicity Values | Not Available |
|---|
| Lethal Dose | Not Available |
|---|
| Carcinogenicity (IARC Classification) | Not Available |
|---|
| Uses/Sources | Not Available |
|---|
| Minimum Risk Level | Not Available |
|---|
| Health Effects | Not Available |
|---|
| Symptoms | Not Available |
|---|
| Treatment | Not Available |
|---|
| Concentrations |
|---|
| Not Available |
|---|
| External Links |
|---|
| DrugBank ID | Not Available |
|---|
| HMDB ID | HMDB0032173 |
|---|
| FooDB ID | FDB008988 |
|---|
| Phenol Explorer ID | Not Available |
|---|
| KNApSAcK ID | Not Available |
|---|
| BiGG ID | Not Available |
|---|
| BioCyc ID | Not Available |
|---|
| METLIN ID | Not Available |
|---|
| PDB ID | Not Available |
|---|
| Wikipedia Link | Not Available |
|---|
| Chemspider ID | 21406545 |
|---|
| ChEBI ID | 174614 |
|---|
| PubChem Compound ID | 24973165 |
|---|
| Kegg Compound ID | Not Available |
|---|
| YMDB ID | Not Available |
|---|
| ECMDB ID | Not Available |
|---|
| References |
|---|
| Synthesis Reference | Not Available |
|---|
| MSDS | Not Available |
|---|
| General References | |
|---|