| Record Information |
|---|
| Version | 1.0 |
|---|
| Creation Date | 2016-05-19 01:54:35 UTC |
|---|
| Update Date | 2016-11-09 01:09:25 UTC |
|---|
| Accession Number | CHEM004762 |
|---|
| Identification |
|---|
| Common Name | ANNATTO, EXTRACT (BIXA ORELLANA L.) |
|---|
| Class | Small Molecule |
|---|
| Description | Annatto ( or ) is an orange-red condiment and food coloring derived from the seeds of the achiote tree (Bixa orellana) native to tropical regions from Mexico to Brazil. It is often used to impart a yellow or orange color to foods, but sometimes also for its flavor and aroma. Its scent is described as "slightly peppery with a hint of nutmeg" and flavor as "slightly nutty, sweet and peppery".The color of annatto comes from various carotenoid pigments, mainly bixin and norbixin, found in the reddish waxy coating of the seeds. The condiment is typically prepared by grinding the seeds to a powder or paste. Similar effects can be obtained by extracting some of the color and flavor principles from the seeds with hot water, oil, or lard, which are then added to the food.Annatto and its extracts are now widely used in an artisanal or industrial scale as a coloring agent in many processed food products, such as cheeses, dairy spreads, butter and margarine, custards, cakes and other baked goods, potatoes, snack foods, breakfast cereals, smoked fish, sausages, and more. In these uses, annatto is a natural alternative to synthetic food coloring compounds, but it has been linked to rare cases of food-related allergies. Annatto is of particular commercial value in the United States because the Food and Drug Administration considers colorants derived from it to be "exempt of certification". |
|---|
| Contaminant Sources | |
|---|
| Contaminant Type | Not Available |
|---|
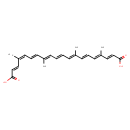
| Chemical Structure | |
|---|
| Synonyms | | Value | Source |
|---|
| Annatto | MeSH | | (4Z,6E,8E,10E,12E,14E,16E,18E)-4,8,13,17-Tetramethylicosa-2,4,6,8,10,12,14,16,18-nonaenedioate | Generator |
|
|---|
| Chemical Formula | C24H28O4 |
|---|
| Average Molecular Mass | 380.484 g/mol |
|---|
| Monoisotopic Mass | 380.199 g/mol |
|---|
| CAS Registry Number | 1393-63-1 |
|---|
| IUPAC Name | (2E,4Z,6E,8E,10E,12E,14E,16E,18E)-4,8,13,17-tetramethylicosa-2,4,6,8,10,12,14,16,18-nonaenedioic acid |
|---|
| Traditional Name | (2E,4Z,6E,8E,10E,12E,14E,16E,18E)-4,8,13,17-tetramethylicosa-2,4,6,8,10,12,14,16,18-nonaenedioic acid |
|---|
| SMILES | C/C(/C=C/C=C(/C)\C=C\C(O)=O)=C\C=C\C=C(/C)\C=C\C=C(/C)\C=C\C(O)=O |
|---|
| InChI Identifier | InChI=1S/C24H28O4/c1-19(11-7-13-21(3)15-17-23(25)26)9-5-6-10-20(2)12-8-14-22(4)16-18-24(27)28/h5-18H,1-4H3,(H,25,26)(H,27,28)/b6-5+,11-7+,12-8+,17-15+,18-16+,19-9+,20-10+,21-13-,22-14+ |
|---|
| InChI Key | ZVKOASAVGLETCT-LRRSNBNMSA-N |
|---|
| Chemical Taxonomy |
|---|
| Description | belongs to the class of organic compounds known as acyclic diterpenoids. These are diterpenoids (compounds made of four consecutive isoprene units) that do not contain a cycle. |
|---|
| Kingdom | Organic compounds |
|---|
| Super Class | Lipids and lipid-like molecules |
|---|
| Class | Prenol lipids |
|---|
| Sub Class | Diterpenoids |
|---|
| Direct Parent | Acyclic diterpenoids |
|---|
| Alternative Parents | |
|---|
| Substituents | - Acyclic diterpenoid
- Long-chain fatty acid
- Branched fatty acid
- Methyl-branched fatty acid
- Dicarboxylic acid or derivatives
- Fatty acyl
- Fatty acid
- Unsaturated fatty acid
- Carboxylic acid derivative
- Carboxylic acid
- Organic oxygen compound
- Carbonyl group
- Organic oxide
- Hydrocarbon derivative
- Organooxygen compound
- Aliphatic acyclic compound
|
|---|
| Molecular Framework | Aliphatic acyclic compounds |
|---|
| External Descriptors | Not Available |
|---|
| Biological Properties |
|---|
| Status | Detected and Not Quantified |
|---|
| Origin | Not Available |
|---|
| Cellular Locations | Not Available |
|---|
| Biofluid Locations | Not Available |
|---|
| Tissue Locations | Not Available |
|---|
| Pathways | Not Available |
|---|
| Applications | Not Available |
|---|
| Biological Roles | Not Available |
|---|
| Chemical Roles | Not Available |
|---|
| Physical Properties |
|---|
| State | Not Available |
|---|
| Appearance | Not Available |
|---|
| Experimental Properties | | Property | Value |
|---|
| Melting Point | Not Available | | Boiling Point | Not Available | | Solubility | Not Available |
|
|---|
| Predicted Properties | |
|---|
| Spectra |
|---|
| Spectra | | Spectrum Type | Description | Splash Key | View |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | splash10-02ti-0229000000-2322a9bf3685724d41e2 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-01q9-0119000000-bdace7c03daee28634a6 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-01w0-1469000000-c45390ff8aad7fc07236 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-0k9b-6981000000-366a0145f78ad333e5ae | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-004i-0009000000-51a9f8bda8016a65d132 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-004i-1009000000-43f52cc2f77325d3b79d | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-07bf-9146000000-3ff70eff236e38ae9608 | Spectrum |
|
|---|
| Toxicity Profile |
|---|
| Route of Exposure | Not Available |
|---|
| Mechanism of Toxicity | Not Available |
|---|
| Metabolism | Not Available |
|---|
| Toxicity Values | Not Available |
|---|
| Lethal Dose | Not Available |
|---|
| Carcinogenicity (IARC Classification) | Not Available |
|---|
| Uses/Sources | Not Available |
|---|
| Minimum Risk Level | Not Available |
|---|
| Health Effects | Not Available |
|---|
| Symptoms | Not Available |
|---|
| Treatment | Not Available |
|---|
| Concentrations |
|---|
| Not Available |
|---|
| External Links |
|---|
| DrugBank ID | Not Available |
|---|
| HMDB ID | Not Available |
|---|
| FooDB ID | Not Available |
|---|
| Phenol Explorer ID | Not Available |
|---|
| KNApSAcK ID | Not Available |
|---|
| BiGG ID | Not Available |
|---|
| BioCyc ID | Not Available |
|---|
| METLIN ID | Not Available |
|---|
| PDB ID | Not Available |
|---|
| Wikipedia Link | Annatto |
|---|
| Chemspider ID | Not Available |
|---|
| ChEBI ID | Not Available |
|---|
| PubChem Compound ID | 6537492 |
|---|
| Kegg Compound ID | Not Available |
|---|
| YMDB ID | Not Available |
|---|
| ECMDB ID | Not Available |
|---|
| References |
|---|
| Synthesis Reference | Not Available |
|---|
| MSDS | Not Available |
|---|
| General References | Not Available |
|---|