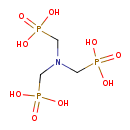
| [Nitrilotris(methylene)]trisphosphonate | Generator |
| (Nitrilotris(methylene))tri-phosphonic acid | HMDB |
| (Nitrilotris(methylene))triphosphonic acid | HMDB |
| (Nitrilotris(methylene))tris-phosphonic acid | HMDB |
| (Nitrilotris(methylene))trisphosphonic acid | HMDB |
| (Nitrilotris(methylene))trisphosphonic acid, sodium salt | HMDB |
| (Nitrilotris-(methylene))tris-phosphoric acid | HMDB |
| 2235-43-0 (Penta-hydrochloride salt) | HMDB |
| 7611-50-9 (Tri-hydrochloride salt) | HMDB |
| Amino, tris(methylene phosphonic acid) | HMDB |
| Aminotri(methylene phosphonic acid) | HMDB |
| Aminotri(methylene phosphonic acid), sodium salt | HMDB |
| Aminotri(methylenephosphonic acid) | HMDB |
| Aminotri(methylphosphonic acid) | HMDB |
| Aminotrimethylene phosphonic acid | HMDB |
| Aminotris(methanephosphonic acid) | HMDB |
| Aminotris(methylphosphonic acid) | HMDB |
| Aminotris(methylphosphonic acid), sodium salts | HMDB |
| Dequest 2000 | HMDB |
| Dequest 2001 | HMDB |
| Dowell L 37 | HMDB |
| Ferrofos 509 | HMDB |
| Nitrilotri(methylphosphonic acid) | HMDB |
| Nitrilotrimethanephosphonic acid | HMDB |
| Nitrilotrimethylenephosphonic acid | HMDB |
| Nitrilotrimethylenetris(phosphonic acid) | HMDB |
| Nitrilotrimethylphosphonic acid | HMDB |
| Nitrilotris(methylene phosphonic acid), sodium salt | HMDB |
| Nitrilotris(methylene)trisphosphonic acid | HMDB |
| Nitrilotris(methylenephosphonic acid) | HMDB |
| Nitrilotris(methylphosphonic acid) | HMDB |
| p,P',p''-(nitrilotris(methylene))tris-phosphonic acid | HMDB |
| Phosphonic acid, (nitrilotris(methylene))tri-, sodium salt | HMDB |
| Phosphonic acid, (nitrilotris(methylene))tris-, sodium salt | HMDB |
| Sodium (nitrilotris(methylene))triphosphonate | HMDB |
| Sodium (nitrilotris(methylene))tris(phosphonate) | HMDB |
| Sodium aminotris(methylenephosphonate) | HMDB |
| Sym-trimethylaminetriphosphonic acid | HMDB |
| Tris(phosphonomethyl)amine | HMDB |
| [Nitrilotris(methylene)]tris(phosphonic acid) | HMDB |
| [Nitrilotris(methylene)]tris-phosphonic acid | HMDB |
| ATMP CPD | HMDB |
| NTMP CPD | HMDB |
| NTMTPA | HMDB |
| Nitrilotris(methyleneiphosphonic acid) | HMDB |
| Pentasodium (nitrilotris(methylene))triphosphonate | HMDB |
| Ammonium (nitrilotris(methylene))triphosphonate | HMDB |
| Heaxammonium (nitrilotris(methylene))triphosphonate | HMDB |
| Tetrammonium (nitrilotris(methylene))triphosphonate | HMDB |
| Trisodium (nitrilotris(methylene))triphosphonic acid | HMDB |
| Hexapotassium (nitrilotris(methylene))triphosphonate | HMDB |
| Pentpotassium (nitrilotris(methylene))triphosphonate | HMDB |
| Triammonium (nitrilotris(methylene))triphosphonate | HMDB |
| Zinc (nitrilotris(methylene))triphosphonate (1:2) | HMDB |
| Aminotris(methylenephosphonic acid) | HMDB |
| Potassium (nitrilotris(methylene))triphosphonate | HMDB |
| Zinc (nitrilotris(methylene))triphosphonate | HMDB |
| {[bis(phosphonomethyl)amino]methyl}phosphonate | HMDB |