| Record Information |
|---|
| Version | 1.0 |
|---|
| Creation Date | 2016-05-19 01:53:05 UTC |
|---|
| Update Date | 2016-11-09 01:09:23 UTC |
|---|
| Accession Number | CHEM004634 |
|---|
| Identification |
|---|
| Common Name | ALKANET ROOT, EXTRACT (ALKANNA TINCTORIA TAUSCH) |
|---|
| Class | Small Molecule |
|---|
| Description | Alkannin is a natural dye that is obtained from the extracts of plants from the borage family Alkanna tinctoria that are found in the south of France. The dye is used as a food coloring and in cosmetics. It is used as a red-brown food additive in regions such as Australia, and is designated in Europe as the E number E103, but is no longer approved for use. Alkannin has a deep red color in a greasy or oily environment and a violet color in an alkaline environment.The chemical structure as a naphthoquinone derivative was first determined by Brockmann in 1936. The enantiomer of alkannin is known as shikonin, and the racemic mixture of the two is known as shikalkin.The enzyme 4-hydroxybenzoate geranyltransferase utilizes geranyl diphosphate and 4-hydroxybenzoate to produce 3-geranyl-4-hydroxybenzoate and diphosphate. These compounds are then used to form alkannin.Alkannin is an antioxidant and has an antimicrobial effect against Staphylococcus aureus and Staphylococcus epidermidis. It is also known to have wound healing, antitumor, and antithrombotic properties.
Shikonin is also found in the Chinese herbal medicine plant Lithospermum erythrorhizon, the red-root gromwell, (紫草 zicao, Pinyin: zǐcǎo). |
|---|
| Contaminant Sources | |
|---|
| Contaminant Type | Not Available |
|---|
| Chemical Structure | |
|---|
| Synonyms | | Value | Source |
|---|
| e103 | ChEMBL | | Alkannin | MeSH |
|
|---|
| Chemical Formula | C16H16O5 |
|---|
| Average Molecular Mass | 288.299 g/mol |
|---|
| Monoisotopic Mass | 288.100 g/mol |
|---|
| CAS Registry Number | 23444-65-7 |
|---|
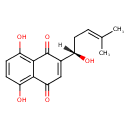
| IUPAC Name | 5,8-dihydroxy-2-[(1S)-1-hydroxy-4-methylpent-3-en-1-yl]-1,4-dihydronaphthalene-1,4-dione |
|---|
| Traditional Name | alkannin |
|---|
| SMILES | [H][C@](O)(CC=C(C)C)C1=CC(=O)C2=C(O)C=CC(O)=C2C1=O |
|---|
| InChI Identifier | InChI=1S/C16H16O5/c1-8(2)3-4-10(17)9-7-13(20)14-11(18)5-6-12(19)15(14)16(9)21/h3,5-7,10,17-19H,4H2,1-2H3/t10-/m0/s1 |
|---|
| InChI Key | NEZONWMXZKDMKF-JTQLQIEISA-N |
|---|
| Chemical Taxonomy |
|---|
| Description | belongs to the class of organic compounds known as naphthoquinones. Naphthoquinones are compounds containing a naphthohydroquinone moiety, which consists of a benzene ring linearly fused to a bezene-1,4-dione (quinone). |
|---|
| Kingdom | Organic compounds |
|---|
| Super Class | Benzenoids |
|---|
| Class | Naphthalenes |
|---|
| Sub Class | Naphthoquinones |
|---|
| Direct Parent | Naphthoquinones |
|---|
| Alternative Parents | |
|---|
| Substituents | - Naphthoquinone
- Aromatic monoterpenoid
- Bicyclic monoterpenoid
- Monoterpenoid
- Aryl ketone
- Quinone
- 1-hydroxy-2-unsubstituted benzenoid
- Vinylogous acid
- Ketone
- Secondary alcohol
- Hydrocarbon derivative
- Alcohol
- Organic oxide
- Organic oxygen compound
- Organooxygen compound
- Aromatic homopolycyclic compound
|
|---|
| Molecular Framework | Aromatic homopolycyclic compounds |
|---|
| External Descriptors | |
|---|
| Biological Properties |
|---|
| Status | Detected and Not Quantified |
|---|
| Origin | Not Available |
|---|
| Cellular Locations | Not Available |
|---|
| Biofluid Locations | Not Available |
|---|
| Tissue Locations | Not Available |
|---|
| Pathways | Not Available |
|---|
| Applications | Not Available |
|---|
| Biological Roles | Not Available |
|---|
| Chemical Roles | Not Available |
|---|
| Physical Properties |
|---|
| State | Not Available |
|---|
| Appearance | Not Available |
|---|
| Experimental Properties | | Property | Value |
|---|
| Melting Point | Not Available | | Boiling Point | Not Available | | Solubility | Not Available |
|
|---|
| Predicted Properties | |
|---|
| Spectra |
|---|
| Spectra | | Spectrum Type | Description | Splash Key | View |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-00dr-0090000000-075012fc8e371dc2176e | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-0fy9-3690000000-91f11e32821de969fc96 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-0gbc-9230000000-192f49a494c02eaf64b3 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-000i-0090000000-d06cd6d33d99ce2f26d7 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-000i-2790000000-649136aee6eabbfaaf17 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-000i-2900000000-bbd3966288658c0b712f | Spectrum |
|
|---|
| Toxicity Profile |
|---|
| Route of Exposure | Not Available |
|---|
| Mechanism of Toxicity | Not Available |
|---|
| Metabolism | Not Available |
|---|
| Toxicity Values | Not Available |
|---|
| Lethal Dose | Not Available |
|---|
| Carcinogenicity (IARC Classification) | Not Available |
|---|
| Uses/Sources | Not Available |
|---|
| Minimum Risk Level | Not Available |
|---|
| Health Effects | Not Available |
|---|
| Symptoms | Not Available |
|---|
| Treatment | Not Available |
|---|
| Concentrations |
|---|
| Not Available |
|---|
| External Links |
|---|
| DrugBank ID | Not Available |
|---|
| HMDB ID | Not Available |
|---|
| FooDB ID | Not Available |
|---|
| Phenol Explorer ID | Not Available |
|---|
| KNApSAcK ID | C00002787 |
|---|
| BiGG ID | Not Available |
|---|
| BioCyc ID | Not Available |
|---|
| METLIN ID | Not Available |
|---|
| PDB ID | Not Available |
|---|
| Wikipedia Link | Alkannin |
|---|
| Chemspider ID | Not Available |
|---|
| ChEBI ID | Not Available |
|---|
| PubChem Compound ID | 72521 |
|---|
| Kegg Compound ID | C10292 |
|---|
| YMDB ID | Not Available |
|---|
| ECMDB ID | Not Available |
|---|
| References |
|---|
| Synthesis Reference | Not Available |
|---|
| MSDS | Not Available |
|---|
| General References | Not Available |
|---|