| Record Information |
|---|
| Version | 1.0 |
|---|
| Creation Date | 2016-05-19 01:52:40 UTC |
|---|
| Update Date | 2016-11-09 01:09:23 UTC |
|---|
| Accession Number | CHEM004613 |
|---|
| Identification |
|---|
| Common Name | 2-ACETYL-2-THIAZOLINE |
|---|
| Class | Small Molecule |
|---|
| Description | 2-Acetyl-4,5-dihydrothiazole is found in alcoholic beverages. 2-Acetyl-4,5-dihydrothiazole is reported in beef broth, roast beef and overpasteurized beer. Roasted meat-like flavour ingredient. |
|---|
| Contaminant Sources | - EAFUS Chemicals
- FooDB Chemicals
- HMDB Contaminants - Feces
|
|---|
| Contaminant Type | Not Available |
|---|
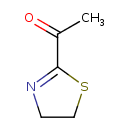
| Chemical Structure | |
|---|
| Synonyms | | Value | Source |
|---|
| 1-(4,5-dihydro-1,3-Thiazol-2-yl)ethanone | HMDB | | 1-(4,5-dihydro-2-Thiazolyl)-ethanone | HMDB | | 1-(4,5-dihydro-2-Thiazolyl)ethanone, 9ci | HMDB | | 2-Acetyl-2-thiazoline | HMDB | | 2-Acetylthiazoline | HMDB | | 2-Thiazoline, 2-acetyl | HMDB | | Acetylthiazoline | HMDB | | FEMA 3817 | HMDB | | Methyl 2-thiazolin-2-yl ketone, 8ci | HMDB |
|
|---|
| Chemical Formula | C5H7NOS |
|---|
| Average Molecular Mass | 129.180 g/mol |
|---|
| Monoisotopic Mass | 129.025 g/mol |
|---|
| CAS Registry Number | 29926-41-8 |
|---|
| IUPAC Name | 1-(4,5-dihydro-1,3-thiazol-2-yl)ethan-1-one |
|---|
| Traditional Name | 1-(4,5-dihydro-1,3-thiazol-2-yl)ethanone |
|---|
| SMILES | CC(=O)C1=NCCS1 |
|---|
| InChI Identifier | InChI=1S/C5H7NOS/c1-4(7)5-6-2-3-8-5/h2-3H2,1H3 |
|---|
| InChI Key | FZOZFDAMVVEZSJ-UHFFFAOYSA-N |
|---|
| Chemical Taxonomy |
|---|
| Description | belongs to the class of organic compounds known as thiazolines. These are heterocyclic compounds containing a five-member unsaturated aliphatic ring with one nitrogen atom, one sulfur atom, three carbon atoms. |
|---|
| Kingdom | Organic compounds |
|---|
| Super Class | Organoheterocyclic compounds |
|---|
| Class | Azolines |
|---|
| Sub Class | Thiazolines |
|---|
| Direct Parent | Thiazolines |
|---|
| Alternative Parents | |
|---|
| Substituents | - Meta-thiazoline
- Ketone
- Azacycle
- Organic 1,3-dipolar compound
- Propargyl-type 1,3-dipolar organic compound
- Organic nitrogen compound
- Organic oxygen compound
- Organopnictogen compound
- Organic oxide
- Hydrocarbon derivative
- Organooxygen compound
- Organonitrogen compound
- Carbonyl group
- Aliphatic heteromonocyclic compound
|
|---|
| Molecular Framework | Aliphatic heteromonocyclic compounds |
|---|
| External Descriptors | Not Available |
|---|
| Biological Properties |
|---|
| Status | Detected and Not Quantified |
|---|
| Origin | Not Available |
|---|
| Cellular Locations | Not Available |
|---|
| Biofluid Locations | Not Available |
|---|
| Tissue Locations | Not Available |
|---|
| Pathways | Not Available |
|---|
| Applications | Not Available |
|---|
| Biological Roles | Not Available |
|---|
| Chemical Roles | Not Available |
|---|
| Physical Properties |
|---|
| State | Not Available |
|---|
| Appearance | Not Available |
|---|
| Experimental Properties | | Property | Value |
|---|
| Melting Point | Not Available | | Boiling Point | Not Available | | Solubility | Not Available |
|
|---|
| Predicted Properties | |
|---|
| Spectra |
|---|
| Spectra | | Spectrum Type | Description | Splash Key | View |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | splash10-0006-9000000000-41b8c24ad55e6d4a0726 | Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | Not Available | Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | Not Available | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-001i-0900000000-6a7fc95ebd43e6f8e45f | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-001i-3900000000-ccd194aa1d8e9a43b377 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-0006-9000000000-cbcf099e196f86821fb5 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-0fb9-3900000000-5f9aa5b87d6493bd201f | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-002r-9300000000-5e2d035bdeecf5d991e8 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-052r-9000000000-9263fff1d71a3bd7f5b8 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-0550-9300000000-ba67bf64b24d2cc557c9 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-001i-9000000000-cf90cabe31b090d8660f | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-0a59-9200000000-818a15a5d8f864bf32ce | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-001i-0900000000-0d28d9db8ec54bdd9fdc | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-001l-9400000000-d6bcc242cd2eef90dead | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-0r03-9000000000-76397b77d3ceed6cbb2d | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum |
|
|---|
| Toxicity Profile |
|---|
| Route of Exposure | Not Available |
|---|
| Mechanism of Toxicity | Not Available |
|---|
| Metabolism | Not Available |
|---|
| Toxicity Values | Not Available |
|---|
| Lethal Dose | Not Available |
|---|
| Carcinogenicity (IARC Classification) | Not Available |
|---|
| Uses/Sources | Not Available |
|---|
| Minimum Risk Level | Not Available |
|---|
| Health Effects | Not Available |
|---|
| Symptoms | Not Available |
|---|
| Treatment | Not Available |
|---|
| Concentrations |
|---|
| Not Available |
|---|
| External Links |
|---|
| DrugBank ID | Not Available |
|---|
| HMDB ID | HMDB0033561 |
|---|
| FooDB ID | FDB011630 |
|---|
| Phenol Explorer ID | Not Available |
|---|
| KNApSAcK ID | Not Available |
|---|
| BiGG ID | Not Available |
|---|
| BioCyc ID | Not Available |
|---|
| METLIN ID | Not Available |
|---|
| PDB ID | Not Available |
|---|
| Wikipedia Link | Not Available |
|---|
| Chemspider ID | 147905 |
|---|
| ChEBI ID | Not Available |
|---|
| PubChem Compound ID | 169110 |
|---|
| Kegg Compound ID | Not Available |
|---|
| YMDB ID | YMDB01448 |
|---|
| ECMDB ID | Not Available |
|---|
| References |
|---|
| Synthesis Reference | Not Available |
|---|
| MSDS | Not Available |
|---|
| General References | |
|---|