| Record Information |
|---|
| Version | 1.0 |
|---|
| Creation Date | 2016-05-19 01:52:38 UTC |
|---|
| Update Date | 2016-11-09 01:09:23 UTC |
|---|
| Accession Number | CHEM004611 |
|---|
| Identification |
|---|
| Common Name | 2-ACETYL-1-PYRROLINE |
|---|
| Class | Small Molecule |
|---|
| Description | A pyrroline that is 1-pyrroline in which the hydrogen at position 2 is replaced by an acetyl group. It is an aroma and flavour compound present in jasmine rice and basmati rice. It is responsible for the 'popcorn' aroma in a large variety of cereal and food products. It is one of the key odourants of the crust of bread and considered to be responsible for the cracker-like odour properties. In bread, it is primarily generated during baking but amounts are influenced by ingredient composition and fermentation conditions. |
|---|
| Contaminant Sources | - EAFUS Chemicals
- FooDB Chemicals
|
|---|
| Contaminant Type | Not Available |
|---|
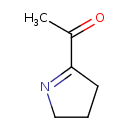
| Chemical Structure | |
|---|
| Synonyms | | Value | Source |
|---|
| 2-Acetyl-4,5-dihydro-3H-pyrrole | ChEBI | | 2AP | ChEBI | | APR | ChEBI | | 1-(3,4-dihydro-2H-Pyrrol-5-yl)ethanone | HMDB | | 1-(3,4-dihydro-2H-Pyrrol-5-yl)ethanone, 9ci | HMDB | | 1-Pyrroline, 2-acetyl | HMDB | | 2-Acetyl-1-pyrolline | HMDB | | 2-Acetyl-1-pyrroline | HMDB, MeSH | | 2-Acetylpyrroline | MeSH, HMDB |
|
|---|
| Chemical Formula | C6H9NO |
|---|
| Average Molecular Mass | 111.142 g/mol |
|---|
| Monoisotopic Mass | 111.068 g/mol |
|---|
| CAS Registry Number | 99583-29-6 |
|---|
| IUPAC Name | 1-(3,4-dihydro-2H-pyrrol-5-yl)ethan-1-one |
|---|
| Traditional Name | 2-acetyl-1-pyrroline |
|---|
| SMILES | CC(=O)C1=NCCC1 |
|---|
| InChI Identifier | InChI=1S/C6H9NO/c1-5(8)6-3-2-4-7-6/h2-4H2,1H3 |
|---|
| InChI Key | DQBQWWSFRPLIAX-UHFFFAOYSA-N |
|---|
| Chemical Taxonomy |
|---|
| Description | belongs to the class of organic compounds known as pyrrolines. Pyrrolines are compounds containing a pyrroline ring, which is a five-member unsaturated aliphatic ring with one nitrogen atom and four carbon atoms. |
|---|
| Kingdom | Organic compounds |
|---|
| Super Class | Organoheterocyclic compounds |
|---|
| Class | Pyrrolines |
|---|
| Sub Class | Not Available |
|---|
| Direct Parent | Pyrrolines |
|---|
| Alternative Parents | |
|---|
| Substituents | - Pyrroline
- Ketimine
- Ketone
- Propargyl-type 1,3-dipolar organic compound
- Organic 1,3-dipolar compound
- Azacycle
- Carbonyl group
- Organopnictogen compound
- Organooxygen compound
- Organonitrogen compound
- Organic oxygen compound
- Organic nitrogen compound
- Imine
- Hydrocarbon derivative
- Organic oxide
- Aliphatic heteromonocyclic compound
|
|---|
| Molecular Framework | Aliphatic heteromonocyclic compounds |
|---|
| External Descriptors | |
|---|
| Biological Properties |
|---|
| Status | Detected and Not Quantified |
|---|
| Origin | Not Available |
|---|
| Cellular Locations | Not Available |
|---|
| Biofluid Locations | Not Available |
|---|
| Tissue Locations | Not Available |
|---|
| Pathways | Not Available |
|---|
| Applications | Not Available |
|---|
| Biological Roles | Not Available |
|---|
| Chemical Roles | Not Available |
|---|
| Physical Properties |
|---|
| State | Not Available |
|---|
| Appearance | Not Available |
|---|
| Experimental Properties | | Property | Value |
|---|
| Melting Point | Not Available | | Boiling Point | Not Available | | Solubility | Not Available |
|
|---|
| Predicted Properties | |
|---|
| Spectra |
|---|
| Spectra | | Spectrum Type | Description | Splash Key | View |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | splash10-014l-9000000000-b2ec2a5a737593af1876 | Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | Not Available | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-03di-5900000000-ba7d8b3068bce1b47b39 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-01ox-9300000000-d40b8a0858de158c3dd1 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-00kf-9000000000-93e30e06cf0f3fac2e9e | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-03di-1900000000-c99599e8eef94b8c0068 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-03di-3900000000-24d2c60642b551195027 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-014l-9000000000-a6046c2688bcfbb4c313 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-03di-1900000000-24735682b0a5560e64ae | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-02t9-9500000000-2e635455b88717ca2f97 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-014l-9000000000-a0c8a0fe2acbc430c438 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-03di-3900000000-7cddab0b1712fe3943e6 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-014l-9100000000-a2ea5be954e40e1ce67b | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-0006-9000000000-efc17d3cfd9d54e8a0c4 | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum |
|
|---|
| Toxicity Profile |
|---|
| Route of Exposure | Not Available |
|---|
| Mechanism of Toxicity | Not Available |
|---|
| Metabolism | Not Available |
|---|
| Toxicity Values | Not Available |
|---|
| Lethal Dose | Not Available |
|---|
| Carcinogenicity (IARC Classification) | Not Available |
|---|
| Uses/Sources | Not Available |
|---|
| Minimum Risk Level | Not Available |
|---|
| Health Effects | Not Available |
|---|
| Symptoms | Not Available |
|---|
| Treatment | Not Available |
|---|
| Concentrations |
|---|
| Not Available |
|---|
| External Links |
|---|
| DrugBank ID | Not Available |
|---|
| HMDB ID | HMDB0031308 |
|---|
| FooDB ID | FDB003364 |
|---|
| Phenol Explorer ID | Not Available |
|---|
| KNApSAcK ID | Not Available |
|---|
| BiGG ID | Not Available |
|---|
| BioCyc ID | Not Available |
|---|
| METLIN ID | Not Available |
|---|
| PDB ID | Not Available |
|---|
| Wikipedia Link | 2-Acetyl-1-pyrroline |
|---|
| Chemspider ID | 456071 |
|---|
| ChEBI ID | 67125 |
|---|
| PubChem Compound ID | 522834 |
|---|
| Kegg Compound ID | Not Available |
|---|
| YMDB ID | YMDB01451 |
|---|
| ECMDB ID | Not Available |
|---|
| References |
|---|
| Synthesis Reference | Not Available |
|---|
| MSDS | Not Available |
|---|
| General References | |
|---|