| Record Information |
|---|
| Version | 1.0 |
|---|
| Creation Date | 2016-05-19 01:52:20 UTC |
|---|
| Update Date | 2016-11-09 01:09:22 UTC |
|---|
| Accession Number | CHEM004585 |
|---|
| Identification |
|---|
| Common Name | ALPHA-ACETOLACTATE DECARBOXYLASE ENZYME PREPARATION FROM BACILLUS SUBTILIS RECOMBINANT |
|---|
| Class | Small Molecule |
|---|
| Description | alpha-Acetolactate decarboxylase (enzyme preparation from bacillus subtilis recombinant) is used as a food additive [EAFUS] ("EAFUS: Everything Added to Food in the United States. [http://www.eafus.com/]") |
|---|
| Contaminant Sources | - EAFUS Chemicals
- FooDB Chemicals
|
|---|
| Contaminant Type | Not Available |
|---|
| Chemical Structure | |
|---|
| Synonyms | | Value | Source |
|---|
| a-Acetolactate decarboxylase (enzyme preparation from bacillus subtilis recombinant) | Generator | | a-Acetolactic acid decarboxylase (enzyme preparation from bacillus subtilis recombinant) | Generator | | alpha-Acetolactic acid decarboxylase (enzyme preparation from bacillus subtilis recombinant) | Generator | | Α-acetolactate decarboxylase (enzyme preparation from bacillus subtilis recombinant) | Generator | | Α-acetolactic acid decarboxylase (enzyme preparation from bacillus subtilis recombinant) | Generator | | N-[4-(4-Indan-2-yl-piperazine-1-sulfonyl)-phenyl]-acetamide | HMDB | | N-(4-{[4-(2,3-dihydro-1H-inden-2-yl)piperazin-1-yl]sulfonyl}phenyl)ethanimidate | HMDB | | N-(4-{[4-(2,3-dihydro-1H-inden-2-yl)piperazin-1-yl]sulphonyl}phenyl)ethanimidate | HMDB | | N-(4-{[4-(2,3-dihydro-1H-inden-2-yl)piperazin-1-yl]sulphonyl}phenyl)ethanimidic acid | HMDB |
|
|---|
| Chemical Formula | C21H25N3O3S |
|---|
| Average Molecular Mass | 399.507 g/mol |
|---|
| Monoisotopic Mass | 399.162 g/mol |
|---|
| CAS Registry Number | 977164-02-5 |
|---|
| IUPAC Name | (Z)-N-(4-{[4-(2,3-dihydro-1H-inden-2-yl)piperazin-1-yl]sulfonyl}phenyl)ethenimidic acid |
|---|
| Traditional Name | (Z)-N-{4-[4-(2,3-dihydro-1H-inden-2-yl)piperazin-1-ylsulfonyl]phenyl}ethenimidic acid |
|---|
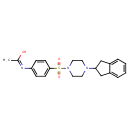
| SMILES | C\C(O)=N\C1=CC=C(C=C1)S(=O)(=O)N1CCN(CC1)C1CC2=CC=CC=C2C1 |
|---|
| InChI Identifier | InChI=1S/C21H25N3O3S/c1-16(25)22-19-6-8-21(9-7-19)28(26,27)24-12-10-23(11-13-24)20-14-17-4-2-3-5-18(17)15-20/h2-9,20H,10-15H2,1H3,(H,22,25) |
|---|
| InChI Key | KYLPQCURLYFTQK-UHFFFAOYSA-N |
|---|
| Chemical Taxonomy |
|---|
| Description | belongs to the class of organic compounds known as benzenesulfonamides. These are organic compounds containing a sulfonamide group that is S-linked to a benzene ring. |
|---|
| Kingdom | Organic compounds |
|---|
| Super Class | Benzenoids |
|---|
| Class | Benzene and substituted derivatives |
|---|
| Sub Class | Benzenesulfonamides |
|---|
| Direct Parent | Benzenesulfonamides |
|---|
| Alternative Parents | |
|---|
| Substituents | - Acetanilide
- Benzenesulfonamide
- Indane
- N-acetylarylamine
- Benzenesulfonyl group
- Anilide
- N-arylamide
- N-alkylpiperazine
- Aralkylamine
- 1,4-diazinane
- Piperazine
- Organosulfonic acid amide
- Acetamide
- Sulfonyl
- Organosulfonic acid or derivatives
- Organic sulfonic acid or derivatives
- Tertiary aliphatic amine
- Tertiary amine
- Secondary carboxylic acid amide
- Amino acid or derivatives
- Carboxamide group
- Azacycle
- Organoheterocyclic compound
- Carboxylic acid derivative
- Hydrocarbon derivative
- Organonitrogen compound
- Organopnictogen compound
- Amine
- Organooxygen compound
- Organosulfur compound
- Carbonyl group
- Organic oxygen compound
- Organic nitrogen compound
- Organic oxide
- Aromatic heteropolycyclic compound
|
|---|
| Molecular Framework | Aromatic heteropolycyclic compounds |
|---|
| External Descriptors | Not Available |
|---|
| Biological Properties |
|---|
| Status | Detected and Not Quantified |
|---|
| Origin | Not Available |
|---|
| Cellular Locations | Not Available |
|---|
| Biofluid Locations | Not Available |
|---|
| Tissue Locations | Not Available |
|---|
| Pathways | Not Available |
|---|
| Applications | Not Available |
|---|
| Biological Roles | Not Available |
|---|
| Chemical Roles | Not Available |
|---|
| Physical Properties |
|---|
| State | Not Available |
|---|
| Appearance | Not Available |
|---|
| Experimental Properties | | Property | Value |
|---|
| Melting Point | Not Available | | Boiling Point | Not Available | | Solubility | Not Available |
|
|---|
| Predicted Properties | |
|---|
| Spectra |
|---|
| Spectra | | Spectrum Type | Description | Splash Key | View |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | splash10-0udi-2792000000-8981344b37ecba5ea8a1 | Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (1 TMS) - 70eV, Positive | splash10-0pbi-6986300000-59b06a0b1106264b8928 | Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | Not Available | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-0zfr-0218900000-1408f6ac04cdf534f306 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-0aor-1928100000-9a9e0b6db581749926c6 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-014i-2911000000-7a7a0c77c15772dcafa9 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-0002-0009000000-650cb35c898bf3a452ab | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-0a4j-1219000000-52072b5a4270ee530533 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-0w2c-6920000000-aad42ce3a2aa2edbed83 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-0k92-0009000000-e9aaf32ab7e9e9dc764d | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-0udi-0009000000-6d235be70f9a35af3f68 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-05o1-1921000000-d138fbbd7b2d99dfdf6f | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-0udi-0100900000-f77022a6a41addc393b6 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-0udi-0311900000-dbeb165c4904d78ecd2f | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-014i-8912000000-b9fb87e4e1d6bb93d5ad | Spectrum |
|
|---|
| Toxicity Profile |
|---|
| Route of Exposure | Not Available |
|---|
| Mechanism of Toxicity | Not Available |
|---|
| Metabolism | Not Available |
|---|
| Toxicity Values | Not Available |
|---|
| Lethal Dose | Not Available |
|---|
| Carcinogenicity (IARC Classification) | Not Available |
|---|
| Uses/Sources | Not Available |
|---|
| Minimum Risk Level | Not Available |
|---|
| Health Effects | Not Available |
|---|
| Symptoms | Not Available |
|---|
| Treatment | Not Available |
|---|
| Concentrations |
|---|
| Not Available |
|---|
| External Links |
|---|
| DrugBank ID | Not Available |
|---|
| HMDB ID | HMDB0032156 |
|---|
| FooDB ID | FDB008885 |
|---|
| Phenol Explorer ID | Not Available |
|---|
| KNApSAcK ID | Not Available |
|---|
| BiGG ID | Not Available |
|---|
| BioCyc ID | Not Available |
|---|
| METLIN ID | Not Available |
|---|
| PDB ID | Not Available |
|---|
| Wikipedia Link | Not Available |
|---|
| Chemspider ID | 977164 |
|---|
| ChEBI ID | Not Available |
|---|
| PubChem Compound ID | 1151802 |
|---|
| Kegg Compound ID | Not Available |
|---|
| YMDB ID | Not Available |
|---|
| ECMDB ID | Not Available |
|---|
| References |
|---|
| Synthesis Reference | Not Available |
|---|
| MSDS | Not Available |
|---|
| General References | |
|---|