| Record Information |
|---|
| Version | 1.0 |
|---|
| Creation Date | 2016-05-19 01:48:40 UTC |
|---|
| Update Date | 2016-11-09 01:09:22 UTC |
|---|
| Accession Number | CHEM004522 |
|---|
| Identification |
|---|
| Common Name | Triazofos |
|---|
| Class | Small Molecule |
|---|
| Description | |
|---|
| Contaminant Sources | - Clean Air Act Chemicals
- My Exposome Chemicals
- STOFF IDENT Compounds
- ToxCast & Tox21 Chemicals
|
|---|
| Contaminant Type | Not Available |
|---|
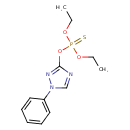
| Chemical Structure | |
|---|
| Synonyms | | Value | Source |
|---|
| 1-Phenyl-1,2,4-triazolyl-3-(O,O-diethylthionophosphate) | ChEBI | | Hostathion | ChEBI | | Methoxone | ChEBI | | O,O-Diethyl O-(1-phenyl-1H-1,2,4-triazol-3-yl) thiophosphate | ChEBI | | Phosphorothioic acid, O,O-diethyl O-(1-phenyl-1H-1,2,4-triazol-3-yl) ester | ChEBI | | Triazofos | ChEBI | | 1-Phenyl-1,2,4-triazolyl-3-(O,O-diethylthionophosphoric acid) | Generator | | O,O-Diethyl O-(1-phenyl-1H-1,2,4-triazol-3-yl) thiophosphoric acid | Generator | | Phosphorothioate, O,O-diethyl O-(1-phenyl-1H-1,2,4-triazol-3-yl) ester | Generator | | Diethoxy-[(1-phenyl-1,2,4-triazol-3-yl)oxy]-sulphanylidene-$l^{5}-phosphane | Generator | | 1-Phenyl-3-(O,O-diethylthiophosphoryl)-1,2,4- triazole | MeSH | | Triazophos | MeSH |
|
|---|
| Chemical Formula | C12H16N3O3PS |
|---|
| Average Molecular Mass | 313.310 g/mol |
|---|
| Monoisotopic Mass | 313.065 g/mol |
|---|
| CAS Registry Number | 24017-47-8 |
|---|
| IUPAC Name | O,O-diethyl O-1-phenyl-1H-1,2,4-triazol-3-yl phosphorothioate |
|---|
| Traditional Name | triazophos |
|---|
| SMILES | CCOP(=S)(OCC)OC1=NN(C=N1)C1=CC=CC=C1 |
|---|
| InChI Identifier | InChI=1S/C12H16N3O3PS/c1-3-16-19(20,17-4-2)18-12-13-10-15(14-12)11-8-6-5-7-9-11/h5-10H,3-4H2,1-2H3 |
|---|
| InChI Key | AMFGTOFWMRQMEM-UHFFFAOYSA-N |
|---|
| Chemical Taxonomy |
|---|
| Description | belongs to the class of organic compounds known as phenyl-1,2,4-triazoles. These are organic compounds containing a 1,2,4-triazole substituted by a phenyl group. |
|---|
| Kingdom | Organic compounds |
|---|
| Super Class | Organoheterocyclic compounds |
|---|
| Class | Azoles |
|---|
| Sub Class | Triazoles |
|---|
| Direct Parent | Phenyl-1,2,4-triazoles |
|---|
| Alternative Parents | |
|---|
| Substituents | - Phenyl-1,2,4-triazole
- Aryl thiophosphate
- Thiophosphate triester
- Monocyclic benzene moiety
- Thiophosphoric acid ester
- Benzenoid
- Organic thiophosphoric acid or derivatives
- Heteroaromatic compound
- Azacycle
- Organic nitrogen compound
- Hydrocarbon derivative
- Organopnictogen compound
- Organooxygen compound
- Organonitrogen compound
- Organic oxygen compound
- Aromatic heteromonocyclic compound
|
|---|
| Molecular Framework | Aromatic heteromonocyclic compounds |
|---|
| External Descriptors | |
|---|
| Biological Properties |
|---|
| Status | Detected and Not Quantified |
|---|
| Origin | Not Available |
|---|
| Cellular Locations | Not Available |
|---|
| Biofluid Locations | Not Available |
|---|
| Tissue Locations | Not Available |
|---|
| Pathways | Not Available |
|---|
| Applications | Not Available |
|---|
| Biological Roles | Not Available |
|---|
| Chemical Roles | Not Available |
|---|
| Physical Properties |
|---|
| State | Not Available |
|---|
| Appearance | Not Available |
|---|
| Experimental Properties | | Property | Value |
|---|
| Melting Point | Not Available | | Boiling Point | Not Available | | Solubility | Not Available |
|
|---|
| Predicted Properties | |
|---|
| Spectra |
|---|
| Spectra | | Spectrum Type | Description | Splash Key | View |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | splash10-0ap0-1690000000-76f246d40827fec3c055 | Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | Not Available | Spectrum | | LC-MS/MS | LC-MS/MS Spectrum - 15V, Positive | splash10-03di-0519000000-0afa8c304e3ddea5705f | Spectrum | | LC-MS/MS | LC-MS/MS Spectrum - 75V, Positive | splash10-03xr-2900000000-eea6a606160a28b26da2 | Spectrum | | LC-MS/MS | LC-MS/MS Spectrum - 90V, Positive | splash10-02tc-5900000000-86c2bd3e4e94ea707a8c | Spectrum | | LC-MS/MS | LC-MS/MS Spectrum - 30V, Positive | splash10-03di-0900000000-daccb74f0194a418e0d1 | Spectrum | | LC-MS/MS | LC-MS/MS Spectrum - 15V, Positive | splash10-03di-0519000000-017a893743eafe4ea90a | Spectrum | | LC-MS/MS | LC-MS/MS Spectrum - 45V, Positive | splash10-03di-0900000000-fb34435b01e06b7457ec | Spectrum | | LC-MS/MS | LC-MS/MS Spectrum - 60V, Positive | splash10-03di-0900000000-cd2bce7d6a7c054b78af | Spectrum | | LC-MS/MS | LC-MS/MS Spectrum - 90V, Positive | splash10-02tc-5900000000-eaa1f2feb222e10960d5 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-03di-2597000000-0496bade75778af59af7 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-0a4i-3940000000-6a51b7b8ee9c20fa7885 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-00kf-9200000000-02d9774a063e4e6e0114 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-02u0-2942000000-f78b9c95142fdf8840a9 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-05gi-0890000000-e91c7df828f1db662ed5 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-004i-0920000000-3074912ab224483230b9 | Spectrum |
|
|---|
| Toxicity Profile |
|---|
| Route of Exposure | Not Available |
|---|
| Mechanism of Toxicity | Not Available |
|---|
| Metabolism | Not Available |
|---|
| Toxicity Values | Not Available |
|---|
| Lethal Dose | Not Available |
|---|
| Carcinogenicity (IARC Classification) | Not Available |
|---|
| Uses/Sources | Not Available |
|---|
| Minimum Risk Level | Not Available |
|---|
| Health Effects | Not Available |
|---|
| Symptoms | Not Available |
|---|
| Treatment | Not Available |
|---|
| Concentrations |
|---|
| Not Available |
|---|
| External Links |
|---|
| DrugBank ID | Not Available |
|---|
| HMDB ID | HMDB0259159 |
|---|
| FooDB ID | Not Available |
|---|
| Phenol Explorer ID | Not Available |
|---|
| KNApSAcK ID | Not Available |
|---|
| BiGG ID | Not Available |
|---|
| BioCyc ID | Not Available |
|---|
| METLIN ID | Not Available |
|---|
| PDB ID | Not Available |
|---|
| Wikipedia Link | Triazofos |
|---|
| Chemspider ID | 29847 |
|---|
| ChEBI ID | 38963 |
|---|
| PubChem Compound ID | Not Available |
|---|
| Kegg Compound ID | C18657 |
|---|
| YMDB ID | Not Available |
|---|
| ECMDB ID | Not Available |
|---|
| References |
|---|
| Synthesis Reference | Not Available |
|---|
| MSDS | Not Available |
|---|
| General References | Not Available |
|---|