| Record Information |
|---|
| Version | 1.0 |
|---|
| Creation Date | 2016-05-19 01:46:48 UTC |
|---|
| Update Date | 2016-11-09 01:09:21 UTC |
|---|
| Accession Number | CHEM004484 |
|---|
| Identification |
|---|
| Common Name | Dialifor |
|---|
| Class | Small Molecule |
|---|
| Description | Not Available |
|---|
| Contaminant Sources | - Clean Air Act Chemicals
- My Exposome Chemicals
- STOFF IDENT Compounds
- ToxCast & Tox21 Chemicals
|
|---|
| Contaminant Type | Not Available |
|---|
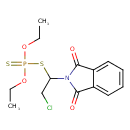
| Chemical Structure | |
|---|
| Synonyms | | Value | Source |
|---|
| O,O-Diethyl {[2-chloro-1-(1,3-dioxo-2,3-dihydro-1H-isoindol-2-yl)ethyl]sulfanyl}phosphonothioic acid | Generator | | O,O-Diethyl {[2-chloro-1-(1,3-dioxo-2,3-dihydro-1H-isoindol-2-yl)ethyl]sulphanyl}phosphonothioate | Generator | | O,O-Diethyl {[2-chloro-1-(1,3-dioxo-2,3-dihydro-1H-isoindol-2-yl)ethyl]sulphanyl}phosphonothioic acid | Generator | | O,O-Diethyl S-(2-chloro-1-phthalimidoethyl) phosphorodithioate | MeSH | | Torak | MeSH | | Torak ec 24 | MeSH | | Dialifor | MeSH |
|
|---|
| Chemical Formula | C14H17ClNO4PS2 |
|---|
| Average Molecular Mass | 393.840 g/mol |
|---|
| Monoisotopic Mass | 393.003 g/mol |
|---|
| CAS Registry Number | 10311-84-9 |
|---|
| IUPAC Name | O,O-diethyl {[2-chloro-1-(1,3-dioxo-2,3-dihydro-1H-isoindol-2-yl)ethyl]sulfanyl}phosphonothioate |
|---|
| Traditional Name | dialifor |
|---|
| SMILES | CCOP(=S)(OCC)SC(CCl)N1C(=O)C2=CC=CC=C2C1=O |
|---|
| InChI Identifier | InChI=1S/C14H17ClNO4PS2/c1-3-19-21(22,20-4-2)23-12(9-15)16-13(17)10-7-5-6-8-11(10)14(16)18/h5-8,12H,3-4,9H2,1-2H3 |
|---|
| InChI Key | MUMQYXACQUZOFP-UHFFFAOYSA-N |
|---|
| Chemical Taxonomy |
|---|
| Description | belongs to the class of organic compounds known as phthalimides. These are aromatic heterocyclic compounds containing a 1,3-dioxoisoindoline moiety. They are imide derivatives of phthalic anhydrides. |
|---|
| Kingdom | Organic compounds |
|---|
| Super Class | Organoheterocyclic compounds |
|---|
| Class | Isoindoles and derivatives |
|---|
| Sub Class | Isoindolines |
|---|
| Direct Parent | Phthalimides |
|---|
| Alternative Parents | |
|---|
| Substituents | - Phthalimide
- Isoindole
- Carboxylic acid imide, n-substituted
- Dithiophosphate o-ester
- Dithiophosphate s-ester
- Benzenoid
- Carboxylic acid imide
- Organic dithiophosphate
- Carboxylic acid derivative
- Organothiophosphorus compound
- Sulfenyl compound
- Azacycle
- Alkyl halide
- Organosulfur compound
- Organooxygen compound
- Organonitrogen compound
- Organochloride
- Organohalogen compound
- Organopnictogen compound
- Organic oxygen compound
- Organic nitrogen compound
- Organic oxide
- Hydrocarbon derivative
- Alkyl chloride
- Aromatic heteropolycyclic compound
|
|---|
| Molecular Framework | Aromatic heteropolycyclic compounds |
|---|
| External Descriptors | |
|---|
| Biological Properties |
|---|
| Status | Detected and Not Quantified |
|---|
| Origin | Not Available |
|---|
| Cellular Locations | Not Available |
|---|
| Biofluid Locations | Not Available |
|---|
| Tissue Locations | Not Available |
|---|
| Pathways | Not Available |
|---|
| Applications | Not Available |
|---|
| Biological Roles | Not Available |
|---|
| Chemical Roles | Not Available |
|---|
| Physical Properties |
|---|
| State | Not Available |
|---|
| Appearance | Not Available |
|---|
| Experimental Properties | | Property | Value |
|---|
| Melting Point | Not Available | | Boiling Point | Not Available | | Solubility | Not Available |
|
|---|
| Predicted Properties | |
|---|
| Spectra |
|---|
| Spectra | | Spectrum Type | Description | Splash Key | View |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | splash10-0aor-1829000000-4b30b94804e742af8582 | Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | Not Available | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-0006-0391000000-7882ed5d62fd52506ef1 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-0a4i-1290000000-b993696255bf02a7b7e3 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-0006-9330000000-6914aee1b71c76301b07 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-052e-0419000000-ea9aa6e689ced15b8b6f | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-0r2i-0469000000-5d4d9cd9b8301c04a0ec | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-0f79-0901000000-b3a6298718678004b553 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-0006-0009000000-62a9a80d57af68835a0c | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-006x-0916000000-6cec2747e887659fc7c9 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-0002-0900000000-986d116cf34911b93b3e | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-0006-0009000000-95e9b0d310d37d515509 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-0r0d-0904000000-dc37546454d4fcccd19d | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-052b-0900000000-2f1b30f631be22e0a2dc | Spectrum |
|
|---|
| Toxicity Profile |
|---|
| Route of Exposure | Not Available |
|---|
| Mechanism of Toxicity | Not Available |
|---|
| Metabolism | Not Available |
|---|
| Toxicity Values | Not Available |
|---|
| Lethal Dose | Not Available |
|---|
| Carcinogenicity (IARC Classification) | Not Available |
|---|
| Uses/Sources | Not Available |
|---|
| Minimum Risk Level | Not Available |
|---|
| Health Effects | Not Available |
|---|
| Symptoms | Not Available |
|---|
| Treatment | Not Available |
|---|
| Concentrations |
|---|
| Not Available |
|---|
| External Links |
|---|
| DrugBank ID | Not Available |
|---|
| HMDB ID | HMDB0251126 |
|---|
| FooDB ID | Not Available |
|---|
| Phenol Explorer ID | Not Available |
|---|
| KNApSAcK ID | Not Available |
|---|
| BiGG ID | Not Available |
|---|
| BioCyc ID | Not Available |
|---|
| METLIN ID | Not Available |
|---|
| PDB ID | Not Available |
|---|
| Wikipedia Link | Not Available |
|---|
| Chemspider ID | 23490 |
|---|
| ChEBI ID | Not Available |
|---|
| PubChem Compound ID | Not Available |
|---|
| Kegg Compound ID | C19028 |
|---|
| YMDB ID | Not Available |
|---|
| ECMDB ID | Not Available |
|---|
| References |
|---|
| Synthesis Reference | Not Available |
|---|
| MSDS | Not Available |
|---|
| General References | Not Available |
|---|