| Record Information |
|---|
| Version | 1.0 |
|---|
| Creation Date | 2016-05-19 01:44:08 UTC |
|---|
| Update Date | 2016-11-09 01:09:20 UTC |
|---|
| Accession Number | CHEM004411 |
|---|
| Identification |
|---|
| Common Name | Sulfotep |
|---|
| Class | Small Molecule |
|---|
| Description | |
|---|
| Contaminant Sources | - Clean Air Act Chemicals
- HPV EPA Chemicals
- My Exposome Chemicals
- STOFF IDENT Compounds
- ToxCast & Tox21 Chemicals
|
|---|
| Contaminant Type | Not Available |
|---|
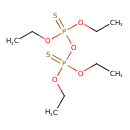
| Chemical Structure | |
|---|
| Synonyms | | Value | Source |
|---|
| Bis(O,O-diethylphosphorothionic) anhydride | ChEBI | | Dithion | ChEBI | | Dithiophos | ChEBI | | O,O,O,O-Tetraethyl dithiopyrophosphate | ChEBI | | Sulfotepp | ChEBI | | TEDP | ChEBI | | TEDTP | ChEBI | | Tetraethyl dithiopyrophosphate | ChEBI | | Thiodiphosphoric acid, tetraethyl ester | ChEBI | | O,O,O,O-Tetraethyl dithiopyrophosphoric acid | Generator | | Sulphotepp | Generator | | Tetraethyl dithiopyrophosphoric acid | Generator | | Thiodiphosphate, tetraethyl ester | Generator | | Sulphotep | Generator | | Diethoxyphosphinothioyloxy-diethoxy-sulphanylidene-$l^{5}-phosphane | Generator | | TETPP | MeSH | | Asymmetric tetraethyl dithiopyrophosphate | MeSH | | Sulfotep | MeSH | | Ethyl thiopyrophosphate | MeSH |
|
|---|
| Chemical Formula | C8H20O5P2S2 |
|---|
| Average Molecular Mass | 322.310 g/mol |
|---|
| Monoisotopic Mass | 322.023 g/mol |
|---|
| CAS Registry Number | 3689-24-5 |
|---|
| IUPAC Name | O,O-diethyl {[diethoxy(sulfanylidene)-λ⁵-phosphanyl]oxy}phosphonothioate |
|---|
| Traditional Name | dithione |
|---|
| SMILES | CCOP(=S)(OCC)OP(=S)(OCC)OCC |
|---|
| InChI Identifier | InChI=1S/C8H20O5P2S2/c1-5-9-14(16,10-6-2)13-15(17,11-7-3)12-8-4/h5-8H2,1-4H3 |
|---|
| InChI Key | XIUROWKZWPIAIB-UHFFFAOYSA-N |
|---|
| Chemical Taxonomy |
|---|
| Description | belongs to the class of organic compounds known as thiophosphoric acid esters. These are organic compounds containing the thiophosphoric acid functional group or a derivative thereof, with the general structure ROP(OR')(OR'')=S, where at least one R-group is an organyl group. |
|---|
| Kingdom | Organic compounds |
|---|
| Super Class | Organic acids and derivatives |
|---|
| Class | Organic thiophosphoric acids and derivatives |
|---|
| Sub Class | Thiophosphoric acid esters |
|---|
| Direct Parent | Thiophosphoric acid esters |
|---|
| Alternative Parents | |
|---|
| Substituents | - Thiophosphoric acid ester
- Organic oxygen compound
- Hydrocarbon derivative
- Organooxygen compound
- Aliphatic acyclic compound
|
|---|
| Molecular Framework | Aliphatic acyclic compounds |
|---|
| External Descriptors | |
|---|
| Biological Properties |
|---|
| Status | Detected and Not Quantified |
|---|
| Origin | Not Available |
|---|
| Cellular Locations | Not Available |
|---|
| Biofluid Locations | Not Available |
|---|
| Tissue Locations | Not Available |
|---|
| Pathways | Not Available |
|---|
| Applications | Not Available |
|---|
| Biological Roles | Not Available |
|---|
| Chemical Roles | Not Available |
|---|
| Physical Properties |
|---|
| State | Not Available |
|---|
| Appearance | Not Available |
|---|
| Experimental Properties | | Property | Value |
|---|
| Melting Point | Not Available | | Boiling Point | Not Available | | Solubility | Not Available |
|
|---|
| Predicted Properties | |
|---|
| Spectra |
|---|
| Spectra | | Spectrum Type | Description | Splash Key | View |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | splash10-02fx-1980000000-8fe064dae1b2f6ae4518 | Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | Not Available | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-00di-1497000000-bf293ef0bcf8926125e3 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-01dl-1790000000-66d53b7ecedbe4865aba | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-00bc-4910000000-7eb346ce0af19f7f3a55 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-00dl-0096000000-91b7c2f88325030d6e6a | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-01ot-0090000000-ff43a2e90a08ae80ba78 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-01pa-0690000000-87b8b36e3083f57e10b5 | Spectrum |
|
|---|
| Toxicity Profile |
|---|
| Route of Exposure | Not Available |
|---|
| Mechanism of Toxicity | Not Available |
|---|
| Metabolism | Not Available |
|---|
| Toxicity Values | Not Available |
|---|
| Lethal Dose | Not Available |
|---|
| Carcinogenicity (IARC Classification) | Not Available |
|---|
| Uses/Sources | Not Available |
|---|
| Minimum Risk Level | Not Available |
|---|
| Health Effects | Not Available |
|---|
| Symptoms | Not Available |
|---|
| Treatment | Not Available |
|---|
| Concentrations |
|---|
| Not Available |
|---|
| External Links |
|---|
| DrugBank ID | DB11497 |
|---|
| HMDB ID | HMDB0258605 |
|---|
| FooDB ID | Not Available |
|---|
| Phenol Explorer ID | Not Available |
|---|
| KNApSAcK ID | Not Available |
|---|
| BiGG ID | Not Available |
|---|
| BioCyc ID | Not Available |
|---|
| METLIN ID | Not Available |
|---|
| PDB ID | Not Available |
|---|
| Wikipedia Link | Sulfotep |
|---|
| Chemspider ID | 18280 |
|---|
| ChEBI ID | 38945 |
|---|
| PubChem Compound ID | Not Available |
|---|
| Kegg Compound ID | Not Available |
|---|
| YMDB ID | Not Available |
|---|
| ECMDB ID | Not Available |
|---|
| References |
|---|
| Synthesis Reference | Not Available |
|---|
| MSDS | Not Available |
|---|
| General References | Not Available |
|---|