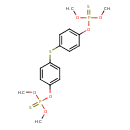
| Synonyms | | Value | Source |
|---|
| Abate | ChEBI | | O,O'-(thiodi-4,1-phenylene) bis(O,O-dimethyl phosphorothioate) | ChEBI | | O,O'-(thiodi-p-phenylene) O,O,o',o'-tetramethyl bis(phosphorothioate) | ChEBI | | O,O,O',o'-tetramethyl O,o'-(sulfanediyldi-4,1-phenylene) bis(phosphorothioate) | ChEBI | | O,O,O',o'-tetramethyl O,o'-(sulfanediyldibenzene-4,1-diyl) bis(thiophosphate) | ChEBI | | O,O,O',o'-tetramethyl O,o'-thiodi-p-phenylene bis(phosphorothioate) | ChEBI | | O,O,O',o'-tetramethyl O,o'-thiodi-p-phenylene diphosphorothioate | ChEBI | | Phosphorothioic acid, O,o'-(thiodi-4,1-phenylene) O,O,o',o'-tetramethyl ester | ChEBI | | Temephos | Kegg | | Abic acid | Generator | | O,O'-(thiodi-4,1-phenylene) bis(O,O-dimethyl phosphorothioic acid) | Generator | | O,O'-(thiodi-p-phenylene) O,O,o',o'-tetramethyl bis(phosphorothioic acid) | Generator | | O,O,O',o'-tetramethyl O,o'-(sulfanediyldi-4,1-phenylene) bis(phosphorothioic acid) | Generator | | O,O,O',o'-tetramethyl O,o'-(sulphanediyldi-4,1-phenylene) bis(phosphorothioate) | Generator | | O,O,O',o'-tetramethyl O,o'-(sulphanediyldi-4,1-phenylene) bis(phosphorothioic acid) | Generator | | O,O,O',o'-tetramethyl O,o'-(sulfanediyldibenzene-4,1-diyl) bis(thiophosphoric acid) | Generator | | O,O,O',o'-tetramethyl O,o'-(sulphanediyldibenzene-4,1-diyl) bis(thiophosphate) | Generator | | O,O,O',o'-tetramethyl O,o'-(sulphanediyldibenzene-4,1-diyl) bis(thiophosphoric acid) | Generator | | O,O,O',o'-tetramethyl O,o'-thiodi-p-phenylene bis(phosphorothioic acid) | Generator | | O,O,O',o'-tetramethyl O,o'-thiodi-p-phenylene diphosphorothioic acid | Generator | | Phosphorothioate, O,o'-(thiodi-4,1-phenylene) O,O,o',o'-tetramethyl ester | Generator | | [4-(4-Dimethoxyphosphinothioyloxyphenyl)sulphanylphenoxy]-dimethoxy-sulphanylidene-$l^{5}-phosphane | Generator | | Difos | MeSH | | Temefos | MeSH, ChEBI |
|
|---|