| Record Information |
|---|
| Version | 1.0 |
|---|
| Creation Date | 2016-05-19 01:43:19 UTC |
|---|
| Update Date | 2016-11-09 01:09:20 UTC |
|---|
| Accession Number | CHEM004387 |
|---|
| Identification |
|---|
| Common Name | Cyanophos |
|---|
| Class | Small Molecule |
|---|
| Description | |
|---|
| Contaminant Sources | - Clean Air Act Chemicals
- My Exposome Chemicals
- STOFF IDENT Compounds
|
|---|
| Contaminant Type | Not Available |
|---|
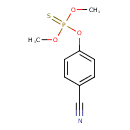
| Chemical Structure | |
|---|
| Synonyms | | Value | Source |
|---|
| 4-(Dimethoxyphosphinothioyloxy)benzonitrile | ChEBI | | Ciafos | ChEBI | | O,O-Dimethyl O-(4-cyanophenyl) thionophosphate | ChEBI | | O,O-Dimethyl-O-p-cyanophenyl phosphorothioate | ChEBI | | O-(4-Cyanophenyl) O,O-dimethyl thiophosphate | ChEBI | | O-p-Cyanophenyl O,O-dimethyl phosphorothioate | ChEBI | | Phosphorothioic acid, O-(4-cyanophenyl) O,O-dimethyl ester | ChEBI | | O,O-Dimethyl O-(4-cyanophenyl) thionophosphoric acid | Generator | | O,O-Dimethyl-O-p-cyanophenyl phosphorothioic acid | Generator | | O-(4-Cyanophenyl) O,O-dimethyl thiophosphoric acid | Generator | | O-p-Cyanophenyl O,O-dimethyl phosphorothioic acid | Generator | | Phosphorothioate, O-(4-cyanophenyl) O,O-dimethyl ester | Generator | | Cyanox | MeSH | | Cyanophos | MeSH |
|
|---|
| Chemical Formula | C9H10NO3PS |
|---|
| Average Molecular Mass | 243.220 g/mol |
|---|
| Monoisotopic Mass | 243.012 g/mol |
|---|
| CAS Registry Number | 2636-26-2 |
|---|
| IUPAC Name | O-4-cyanophenyl O,O-dimethyl phosphorothioate |
|---|
| Traditional Name | cyanox |
|---|
| SMILES | COP(=S)(OC)OC1=CC=C(C=C1)C#N |
|---|
| InChI Identifier | InChI=1S/C9H10NO3PS/c1-11-14(15,12-2)13-9-5-3-8(7-10)4-6-9/h3-6H,1-2H3 |
|---|
| InChI Key | SCKHCCSZFPSHGR-UHFFFAOYSA-N |
|---|
| Chemical Taxonomy |
|---|
| Description | belongs to the class of organic compounds known as phenyl thiophosphates. These are organothiophosphorus compounds that contain a thiophosphoric acid O-esterified with a phenyl group. |
|---|
| Kingdom | Organic compounds |
|---|
| Super Class | Organic acids and derivatives |
|---|
| Class | Organic thiophosphoric acids and derivatives |
|---|
| Sub Class | Thiophosphoric acid esters |
|---|
| Direct Parent | Phenyl thiophosphates |
|---|
| Alternative Parents | |
|---|
| Substituents | - Phenyl thiophosphate
- Phenoxy compound
- Thiophosphate triester
- Benzonitrile
- Benzenoid
- Monocyclic benzene moiety
- Nitrile
- Carbonitrile
- Organic nitrogen compound
- Organic oxygen compound
- Organopnictogen compound
- Hydrocarbon derivative
- Organooxygen compound
- Organonitrogen compound
- Aromatic homomonocyclic compound
|
|---|
| Molecular Framework | Aromatic homomonocyclic compounds |
|---|
| External Descriptors | |
|---|
| Biological Properties |
|---|
| Status | Detected and Not Quantified |
|---|
| Origin | Not Available |
|---|
| Cellular Locations | Not Available |
|---|
| Biofluid Locations | Not Available |
|---|
| Tissue Locations | Not Available |
|---|
| Pathways | Not Available |
|---|
| Applications | Not Available |
|---|
| Biological Roles | Not Available |
|---|
| Chemical Roles | Not Available |
|---|
| Physical Properties |
|---|
| State | Not Available |
|---|
| Appearance | Not Available |
|---|
| Experimental Properties | | Property | Value |
|---|
| Melting Point | Not Available | | Boiling Point | Not Available | | Solubility | Not Available |
|
|---|
| Predicted Properties | |
|---|
| Spectra |
|---|
| Spectra | | Spectrum Type | Description | Splash Key | View |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | splash10-046u-6690000000-2900208ca724c1f44891 | Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | Not Available | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-0006-0090000000-3291d8c67e3b837514dc | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-0006-0090000000-fa4cedfe5d940b6c3d1f | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-0006-6390000000-e8aedc16349dc055c215 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-0006-0090000000-2a87e5f594d6d2a51fea | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-0006-0090000000-35a7c9a44d909f98b81a | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-0006-7590000000-df36c892b4020a79ca40 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-0006-0290000000-90e6baa4347eda4294a4 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-006x-0590000000-7b07ee2dcf86dc0fa7c3 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-0uk9-8900000000-3e9487f6c8c7e7397d17 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-0006-0090000000-fd1509cd8bfa5c51abc5 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-0006-0090000000-fd1509cd8bfa5c51abc5 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-014l-9700000000-ad743aece92b12e4bb91 | Spectrum |
|
|---|
| Toxicity Profile |
|---|
| Route of Exposure | Not Available |
|---|
| Mechanism of Toxicity | Not Available |
|---|
| Metabolism | Not Available |
|---|
| Toxicity Values | Not Available |
|---|
| Lethal Dose | Not Available |
|---|
| Carcinogenicity (IARC Classification) | Not Available |
|---|
| Uses/Sources | Not Available |
|---|
| Minimum Risk Level | Not Available |
|---|
| Health Effects | Not Available |
|---|
| Symptoms | Not Available |
|---|
| Treatment | Not Available |
|---|
| Concentrations |
|---|
| Not Available |
|---|
| External Links |
|---|
| DrugBank ID | Not Available |
|---|
| HMDB ID | HMDB0250614 |
|---|
| FooDB ID | Not Available |
|---|
| Phenol Explorer ID | Not Available |
|---|
| KNApSAcK ID | Not Available |
|---|
| BiGG ID | Not Available |
|---|
| BioCyc ID | Not Available |
|---|
| METLIN ID | Not Available |
|---|
| PDB ID | Not Available |
|---|
| Wikipedia Link | Cyanophos |
|---|
| Chemspider ID | 16569 |
|---|
| ChEBI ID | 38621 |
|---|
| PubChem Compound ID | Not Available |
|---|
| Kegg Compound ID | C18397 |
|---|
| YMDB ID | Not Available |
|---|
| ECMDB ID | Not Available |
|---|
| References |
|---|
| Synthesis Reference | Not Available |
|---|
| MSDS | Not Available |
|---|
| General References | Not Available |
|---|