| Record Information |
|---|
| Version | 1.0 |
|---|
| Creation Date | 2016-05-19 01:43:01 UTC |
|---|
| Update Date | 2016-11-09 01:09:20 UTC |
|---|
| Accession Number | CHEM004379 |
|---|
| Identification |
|---|
| Common Name | Chinomethionat |
|---|
| Class | Small Molecule |
|---|
| Description | A dithioloquinoxaline that results from the formal condensation of 6-methylquinoxaline-2,3-dithiol with phosgene. It has been used as a fungicide and acaricide for the control of mites and powdery mildew on citrus, vegetables, and walnuts, but is not approved for use in the EU. |
|---|
| Contaminant Sources | - Clean Air Act Chemicals
- HPV EPA Chemicals
- My Exposome Chemicals
- STOFF IDENT Compounds
- ToxCast & Tox21 Chemicals
|
|---|
| Contaminant Type | Not Available |
|---|
| Chemical Structure | |
|---|
| Synonyms | | Value | Source |
|---|
| 6-Methyl-2,3-quinoxalinedithiol cyclic dithiocarbonate | ChEBI | | 6-Methyl-2,3-quinoxalinedithiol cyclic S,S-dithiocarbonate | ChEBI | | Chinomethionat | ChEBI | | Chinomethionate | ChEBI | | Morestan | ChEBI | | Oxythioquinox | ChEBI | | S,S-(6-Methylquinoxaline-2,3-diyl) dithiocarbonate | ChEBI | | 6-Methyl-2,3-quinoxalinedithiol cyclic dithiocarbonic acid | Generator | | 6-Methyl-2,3-quinoxalinedithiol cyclic S,S-dithiocarbonic acid | Generator | | Chinomethionic acid | Generator | | S,S-(6-Methylquinoxaline-2,3-diyl) dithiocarbonic acid | Generator | | Quinomethionic acid | Generator | | 6-Methyl-2,3-quinoxalinedithiol cyclic carbonate | MeSH | | Quinomethionate | MeSH, KEGG | | 6-Methyl-2-oxo-1,3-dithio(4,5-b)quinoxaline | MeSH |
|
|---|
| Chemical Formula | C10H6N2OS2 |
|---|
| Average Molecular Mass | 234.290 g/mol |
|---|
| Monoisotopic Mass | 233.992 g/mol |
|---|
| CAS Registry Number | 2439-01-2 |
|---|
| IUPAC Name | 6-methyl-2H-[1,3]dithiolo[4,5-b]quinoxalin-2-one |
|---|
| Traditional Name | joust |
|---|
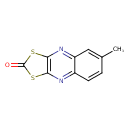
| SMILES | CC1=CC2=NC3=C(SC(=O)S3)N=C2C=C1 |
|---|
| InChI Identifier | InChI=1S/C10H6N2OS2/c1-5-2-3-6-7(4-5)12-9-8(11-6)14-10(13)15-9/h2-4H,1H3 |
|---|
| InChI Key | FBQQHUGEACOBDN-UHFFFAOYSA-N |
|---|
| Chemical Taxonomy |
|---|
| Description | belongs to the class of organic compounds known as quinoxalines. Quinoxalines are compounds containing a quinoxaline moiety, a bicyclic heterocycle made up of a benzene ring fused to a pyrazine ring. |
|---|
| Kingdom | Organic compounds |
|---|
| Super Class | Organoheterocyclic compounds |
|---|
| Class | Diazanaphthalenes |
|---|
| Sub Class | Benzodiazines |
|---|
| Direct Parent | Quinoxalines |
|---|
| Alternative Parents | |
|---|
| Substituents | - Quinoxaline
- Benzenoid
- Pyrazine
- Heteroaromatic compound
- Dithiole
- 1,3-dithiole
- Azacycle
- Organic nitrogen compound
- Organic oxygen compound
- Organopnictogen compound
- Organic oxide
- Hydrocarbon derivative
- Organooxygen compound
- Organonitrogen compound
- Aromatic heteropolycyclic compound
|
|---|
| Molecular Framework | Aromatic heteropolycyclic compounds |
|---|
| External Descriptors | |
|---|
| Biological Properties |
|---|
| Status | Detected and Not Quantified |
|---|
| Origin | Not Available |
|---|
| Cellular Locations | Not Available |
|---|
| Biofluid Locations | Not Available |
|---|
| Tissue Locations | Not Available |
|---|
| Pathways | Not Available |
|---|
| Applications | Not Available |
|---|
| Biological Roles | Not Available |
|---|
| Chemical Roles | Not Available |
|---|
| Physical Properties |
|---|
| State | Not Available |
|---|
| Appearance | Not Available |
|---|
| Experimental Properties | | Property | Value |
|---|
| Melting Point | Not Available | | Boiling Point | Not Available | | Solubility | Not Available |
|
|---|
| Predicted Properties | |
|---|
| Spectra |
|---|
| Spectra | | Spectrum Type | Description | Splash Key | View |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-001i-0090000000-b0b58c4e467b3d6a8768 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-001i-0090000000-74df41242660bd101776 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-001i-0090000000-ea9484413b9d4cdde65d | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-001i-0090000000-2049fcae9515674f0623 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-00di-0900000000-8edd66f7ab34eb93a9ff | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-00di-0900000000-1f497459bf4a8cce8119 | Spectrum |
|
|---|
| Toxicity Profile |
|---|
| Route of Exposure | Not Available |
|---|
| Mechanism of Toxicity | Not Available |
|---|
| Metabolism | Not Available |
|---|
| Toxicity Values | Not Available |
|---|
| Lethal Dose | Not Available |
|---|
| Carcinogenicity (IARC Classification) | Not Available |
|---|
| Uses/Sources | Not Available |
|---|
| Minimum Risk Level | Not Available |
|---|
| Health Effects | Not Available |
|---|
| Symptoms | Not Available |
|---|
| Treatment | Not Available |
|---|
| Concentrations |
|---|
| Not Available |
|---|
| External Links |
|---|
| DrugBank ID | Not Available |
|---|
| HMDB ID | Not Available |
|---|
| FooDB ID | Not Available |
|---|
| Phenol Explorer ID | Not Available |
|---|
| KNApSAcK ID | Not Available |
|---|
| BiGG ID | Not Available |
|---|
| BioCyc ID | Not Available |
|---|
| METLIN ID | Not Available |
|---|
| PDB ID | Not Available |
|---|
| Wikipedia Link | Not Available |
|---|
| Chemspider ID | Not Available |
|---|
| ChEBI ID | 34620 |
|---|
| PubChem Compound ID | 17109 |
|---|
| Kegg Compound ID | C14514 |
|---|
| YMDB ID | Not Available |
|---|
| ECMDB ID | Not Available |
|---|
| References |
|---|
| Synthesis Reference | Not Available |
|---|
| MSDS | Not Available |
|---|
| General References | |
|---|