| Record Information |
|---|
| Version | 1.0 |
|---|
| Creation Date | 2016-05-19 01:42:57 UTC |
|---|
| Update Date | 2016-11-09 01:09:20 UTC |
|---|
| Accession Number | CHEM004377 |
|---|
| Identification |
|---|
| Common Name | Prothoate |
|---|
| Class | Small Molecule |
|---|
| Description | Prothoate is an organothiophosphate insecticide also used as an acaricide.
It is listed as an extremely hazardous substance according to the U.S. Emergency Planning and Community Right-to-Know Act. |
|---|
| Contaminant Sources | - Clean Air Act Chemicals
- My Exposome Chemicals
|
|---|
| Contaminant Type | Not Available |
|---|
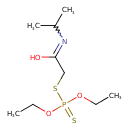
| Chemical Structure | |
|---|
| Synonyms | | Value | Source |
|---|
| Prothoic acid | Generator | | 2-{[diethoxy(sulfanylidene)-λ⁵-phosphanyl]sulfanyl}-N-(propan-2-yl)ethanimidate | Generator | | 2-{[diethoxy(sulphanylidene)-λ⁵-phosphanyl]sulphanyl}-N-(propan-2-yl)ethanimidate | Generator | | 2-{[diethoxy(sulphanylidene)-λ⁵-phosphanyl]sulphanyl}-N-(propan-2-yl)ethanimidic acid | Generator |
|
|---|
| Chemical Formula | C9H20NO3PS2 |
|---|
| Average Molecular Mass | 285.360 g/mol |
|---|
| Monoisotopic Mass | 285.062 g/mol |
|---|
| CAS Registry Number | 2275-18-5 |
|---|
| IUPAC Name | 2-{[diethoxy(sulfanylidene)-λ⁵-phosphanyl]sulfanyl}-N-(propan-2-yl)ethanimidic acid |
|---|
| Traditional Name | 2-{[diethoxy(sulfanylidene)-λ⁵-phosphanyl]sulfanyl}-N-isopropylethanimidic acid |
|---|
| SMILES | CCOP(=S)(OCC)SCC(O)=NC(C)C |
|---|
| InChI Identifier | InChI=1S/C9H20NO3PS2/c1-5-12-14(15,13-6-2)16-7-9(11)10-8(3)4/h8H,5-7H2,1-4H3,(H,10,11) |
|---|
| InChI Key | QTXHFDHVLBDJIO-UHFFFAOYSA-N |
|---|
| Chemical Taxonomy |
|---|
| Description | belongs to the class of organic compounds known as dithiophosphate o-esters. These are o-ester derivatives of dithiophosphates, with the general structure RSP(O)(O)=S (R = organyl group). |
|---|
| Kingdom | Organic compounds |
|---|
| Super Class | Organic acids and derivatives |
|---|
| Class | Organic dithiophosphoric acids and derivatives |
|---|
| Sub Class | Dithiophosphate O-esters |
|---|
| Direct Parent | Dithiophosphate O-esters |
|---|
| Alternative Parents | |
|---|
| Substituents | - Dithiophosphate s-ester
- Dithiophosphate o-ester
- Carboximidic acid
- Organic 1,3-dipolar compound
- Propargyl-type 1,3-dipolar organic compound
- Sulfenyl compound
- Organothiophosphorus compound
- Carboximidic acid derivative
- Organopnictogen compound
- Hydrocarbon derivative
- Organosulfur compound
- Organooxygen compound
- Organonitrogen compound
- Organic oxygen compound
- Organic nitrogen compound
- Aliphatic acyclic compound
|
|---|
| Molecular Framework | Aliphatic acyclic compounds |
|---|
| External Descriptors | |
|---|
| Biological Properties |
|---|
| Status | Detected and Not Quantified |
|---|
| Origin | Not Available |
|---|
| Cellular Locations | Not Available |
|---|
| Biofluid Locations | Not Available |
|---|
| Tissue Locations | Not Available |
|---|
| Pathways | Not Available |
|---|
| Applications | Not Available |
|---|
| Biological Roles | Not Available |
|---|
| Chemical Roles | Not Available |
|---|
| Physical Properties |
|---|
| State | Not Available |
|---|
| Appearance | Not Available |
|---|
| Experimental Properties | | Property | Value |
|---|
| Melting Point | Not Available | | Boiling Point | Not Available | | Solubility | Not Available |
|
|---|
| Predicted Properties | |
|---|
| Spectra |
|---|
| Spectra | | Spectrum Type | Description | Splash Key | View |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-001r-2920000000-f0af00c0e1e718f35de5 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-0aba-5930000000-c93df858b55b158b90e1 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-0a4i-9200000000-ad794d784d6d3a9232e1 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-0f89-0490000000-e2b1bf3d53cb4dda406d | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-0a59-2890000000-10d88b59b19281cdfc17 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-0a4i-7980000000-59d44d11ee62724b7bc5 | Spectrum |
|
|---|
| Toxicity Profile |
|---|
| Route of Exposure | Not Available |
|---|
| Mechanism of Toxicity | Not Available |
|---|
| Metabolism | Not Available |
|---|
| Toxicity Values | Not Available |
|---|
| Lethal Dose | Not Available |
|---|
| Carcinogenicity (IARC Classification) | Not Available |
|---|
| Uses/Sources | Not Available |
|---|
| Minimum Risk Level | Not Available |
|---|
| Health Effects | Not Available |
|---|
| Symptoms | Not Available |
|---|
| Treatment | Not Available |
|---|
| Concentrations |
|---|
| Not Available |
|---|
| External Links |
|---|
| DrugBank ID | Not Available |
|---|
| HMDB ID | Not Available |
|---|
| FooDB ID | Not Available |
|---|
| Phenol Explorer ID | Not Available |
|---|
| KNApSAcK ID | Not Available |
|---|
| BiGG ID | Not Available |
|---|
| BioCyc ID | Not Available |
|---|
| METLIN ID | Not Available |
|---|
| PDB ID | Not Available |
|---|
| Wikipedia Link | Prothoate |
|---|
| Chemspider ID | Not Available |
|---|
| ChEBI ID | Not Available |
|---|
| PubChem Compound ID | 16774 |
|---|
| Kegg Compound ID | C19014 |
|---|
| YMDB ID | Not Available |
|---|
| ECMDB ID | Not Available |
|---|
| References |
|---|
| Synthesis Reference | Not Available |
|---|
| MSDS | Not Available |
|---|
| General References | Not Available |
|---|