| Record Information |
|---|
| Version | 1.0 |
|---|
| Creation Date | 2016-05-19 01:37:22 UTC |
|---|
| Update Date | 2016-11-09 01:09:18 UTC |
|---|
| Accession Number | CHEM004204 |
|---|
| Identification |
|---|
| Common Name | Nabam |
|---|
| Class | Small Molecule |
|---|
| Description | A dithiocarbamate salt that is the disodium salt of ethylenebis(dithiocarbamic acid). A fungicide, algicide and bactericide used on various crops including on cotton, capsicums, onions and rice crops, it is considered to be a carcinogen, so is not licensed for use within the European Union. Mixing nabam with zinc sulfate affords the fungicide zineb. |
|---|
| Contaminant Sources | - Clean Air Act Chemicals
- EAFUS Chemicals
- FooDB Chemicals
- HPV EPA Chemicals
- ToxCast & Tox21 Chemicals
|
|---|
| Contaminant Type | Not Available |
|---|
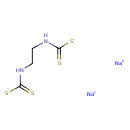
| Chemical Structure | |
|---|
| Synonyms | | Value | Source |
|---|
| 1,2-Ethanediylbiscarbamodithioic acid disodium salt | ChEBI | | Di-natrium-aethylenbisdithiocarbamat | ChEBI | | Dinatrium-(N,n'-aethylen-bis(dithiocarbamat)) | ChEBI | | Disodium ethylene-1,2-bisdithiocarbamate | ChEBI | | Disodium ethylenebis(dithiocarbamate) | ChEBI | | Disodium N,n'-1,2-ethanediylbis(carbamodithioate) | ChEBI | | Disodium N,n'-ethane-1,2-diyldicarbamodithioate | ChEBI | | Dithane a-40 | ChEBI | | N,N'-ethylene bis(dithiocarbamate de sodium) | ChEBI | | Nabame | ChEBI | | Parzate | ChEBI | | Sodium ethylenebis(dithiocarbamate) | ChEBI | | Spring-bak | ChEBI | | 1,2-Ethanediylbiscarbamodithioate disodium salt | Generator | | Disodium ethylene-1,2-bisdithiocarbamic acid | Generator | | Disodium ethylenebis(dithiocarbamic acid) | Generator | | Disodium N,n'-1,2-ethanediylbis(carbamodithioic acid) | Generator | | Disodium N,n'-ethane-1,2-diyldicarbamodithioic acid | Generator | | N,N'-ethylene bis(dithiocarbamic acid de sodium) | Generator | | Parzic acid | Generator | | Sodium ethylenebis(dithiocarbamic acid) | Generator | | Amobam | HMDB | | Carbamic acid, ethylenebis(dithio-, disodium salt | HMDB | | Carbamodithioic acid, 1,2-ethanediylbis-, disodium | HMDB | | Carbamodithioic acid, 1,2-ethanediylbis-, disodium salt | HMDB | | Chem bam | HMDB | | Dinatrium-(N,n'-ethyleen-bis(dithiocarbamaat)) | HMDB | | Disodium 1,2-ethanediylbis (carbamodithioate) | HMDB | | Disodium ethane-1,2-diylbis(dithiocarbamate) | HMDB | | Disodium ethylenebisdithiocarbamate | HMDB | | Dithane a40 | HMDB | | Dithane D-14 | HMDB | | Dithane D-14 (discontinued) | HMDB | | Dithiane D-14 | HMDB | | DSE | HMDB | | Ebdc, disodium salt | HMDB | | Ethylen-bis-dithiokarbaman sodny | HMDB | | Ethylenebis(dithiocarbamate) sodium | HMDB | | Ethylenebis(dithiocarbamate), disodium salt | HMDB | | Ethylenebis(dithiocarbamic acid) disodium salt | HMDB | | Ethylenebisdithiocarbamate, disodium | HMDB | | N,N'-etilen-bis(ditiocarbammato) di sodio | HMDB | | Nabam, ammonium salt | HMDB | | Nabam, bsi, iso | HMDB | | Nabam, calcium salt (1:1) | HMDB | | Nabam, diammonium salt | HMDB | | Nabam, dipotassium salt | HMDB | | Nabam, disodium salt | HMDB | | Nabam, iron salt | HMDB | | Nabam, potassium salt | HMDB | | Nabam, sodium salt | HMDB | | Nabasan | HMDB | | Nafun ipo | HMDB | | Parzate liquid | HMDB | | X-Spor | HMDB |
|
|---|
| Chemical Formula | C4H6N2Na2S4 |
|---|
| Average Molecular Mass | 256.343 g/mol |
|---|
| Monoisotopic Mass | 255.921 g/mol |
|---|
| CAS Registry Number | 142-59-6 |
|---|
| IUPAC Name | disodium ({2-[(sulfanidylmethanethioyl)amino]ethyl}carbamothioyl)sulfanide |
|---|
| Traditional Name | disodium ({2-[(sulfanidylmethanethioyl)amino]ethyl}carbamothioyl)sulfanide |
|---|
| SMILES | [Na+].[Na+].[S-]C(=S)NCCNC([S-])=S |
|---|
| InChI Identifier | InChI=1S/C4H8N2S4.2Na/c7-3(8)5-1-2-6-4(9)10;;/h1-2H2,(H2,5,7,8)(H2,6,9,10);;/q;2*+1/p-2 |
|---|
| InChI Key | UQJQVUOTMVCFHX-UHFFFAOYSA-L |
|---|
| Chemical Taxonomy |
|---|
| Description | belongs to the class of organic compounds known as organic alkali metal salts. These are organic salts of an alkali metal. The alkali metal atom is usually in its ionic form. |
|---|
| Kingdom | Organic compounds |
|---|
| Super Class | Organic salts |
|---|
| Class | Organic metal salts |
|---|
| Sub Class | Organic alkali metal salts |
|---|
| Direct Parent | Organic alkali metal salts |
|---|
| Alternative Parents | |
|---|
| Substituents | - Organic alkali metal salt
- Organic 1,3-dipolar compound
- Propargyl-type 1,3-dipolar organic compound
- Organic nitrogen compound
- Organopnictogen compound
- Hydrocarbon derivative
- Organic sodium salt
- Organosulfur compound
- Organonitrogen compound
- Aliphatic acyclic compound
|
|---|
| Molecular Framework | Aliphatic acyclic compounds |
|---|
| External Descriptors | |
|---|
| Biological Properties |
|---|
| Status | Detected and Not Quantified |
|---|
| Origin | Not Available |
|---|
| Cellular Locations | Not Available |
|---|
| Biofluid Locations | Not Available |
|---|
| Tissue Locations | Not Available |
|---|
| Pathways | Not Available |
|---|
| Applications | Not Available |
|---|
| Biological Roles | Not Available |
|---|
| Chemical Roles | Not Available |
|---|
| Physical Properties |
|---|
| State | Not Available |
|---|
| Appearance | Not Available |
|---|
| Experimental Properties | | Property | Value |
|---|
| Melting Point | Not Available | | Boiling Point | Not Available | | Solubility | Not Available |
|
|---|
| Predicted Properties | |
|---|
| Spectra |
|---|
| Spectra | | Spectrum Type | Description | Splash Key | View |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | splash10-004i-7900000000-7c1cffaeb5d7a4c6cc95 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-0a4i-3190000000-493d02df2322a17f4453 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-0a4i-1790000000-11abdc66a716323b9eac | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-0002-9100000000-9b11c8495ac5a2747256 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-0udi-0090000000-d90fb688c5d69b45c694 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-0udi-0090000000-d90fb688c5d69b45c694 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-0udi-0090000000-d90fb688c5d69b45c694 | Spectrum |
|
|---|
| Toxicity Profile |
|---|
| Route of Exposure | Not Available |
|---|
| Mechanism of Toxicity | Not Available |
|---|
| Metabolism | Not Available |
|---|
| Toxicity Values | Not Available |
|---|
| Lethal Dose | Not Available |
|---|
| Carcinogenicity (IARC Classification) | Not Available |
|---|
| Uses/Sources | Not Available |
|---|
| Minimum Risk Level | Not Available |
|---|
| Health Effects | Not Available |
|---|
| Symptoms | Not Available |
|---|
| Treatment | Not Available |
|---|
| Concentrations |
|---|
| Not Available |
|---|
| External Links |
|---|
| DrugBank ID | Not Available |
|---|
| HMDB ID | HMDB0031224 |
|---|
| FooDB ID | FDB003248 |
|---|
| Phenol Explorer ID | Not Available |
|---|
| KNApSAcK ID | Not Available |
|---|
| BiGG ID | Not Available |
|---|
| BioCyc ID | Not Available |
|---|
| METLIN ID | Not Available |
|---|
| PDB ID | Not Available |
|---|
| Wikipedia Link | Not Available |
|---|
| Chemspider ID | Not Available |
|---|
| ChEBI ID | 81934 |
|---|
| PubChem Compound ID | 8891 |
|---|
| Kegg Compound ID | C18748 |
|---|
| YMDB ID | Not Available |
|---|
| ECMDB ID | Not Available |
|---|
| References |
|---|
| Synthesis Reference | Not Available |
|---|
| MSDS | Not Available |
|---|
| General References | |
|---|