| Record Information |
|---|
| Version | 1.0 |
|---|
| Creation Date | 2016-05-19 01:32:55 UTC |
|---|
| Update Date | 2016-10-28 10:01:04 UTC |
|---|
| Accession Number | CHEM004031 |
|---|
| Identification |
|---|
| Common Name | Ethane, 1,1,2-trichloro-1,2,2,-trifluoro- |
|---|
| Class | Small Molecule |
|---|
| Description | It is used in freezing of foods
CFC-113 is a very unreactive chlorofluorocarbon, that will stay in the atmosphere for a great deal of time if it is released. CFC-113 will stay in the atmosphere long enough that it will cycle out of the troposphere and into the stratosphere. In the stratosphere, CFC-113 can be broken up by ultraviolet radiation, creating chlorine radicals, which can in turn react with ozone molecules to form molecular oxygen (O2), leading to the overall depletion of stratospheric ozone. The amount of CFC-113 in the atmosphere has stayed relatively stable, at about 80 parts per trillion, since the early 1990s.; CFC-113 was one of the many forms of CFCs that were made to eliminate toxic and flammable substances in the areas that they were used. It has been used as a cooling agent in refrigerants and air conditioners, aerosol propellant, and a cleansing agent for electrical and electronic components. CFC-113 is one of the three most popular CFCs, along with CFC-11 and CFC-12 and saw much use in its time. CFC-113 has a unique property that makes it perfect for cooling systems. When it is in a gas form and compressed, it heats up, when it is expanded, it cools. This makes them ideal for the vapor compression cycle systems. They were also very desirable because of their low toxicity, non-flammability, thermophysical properties, and normal boiling point. CFC-113 also has a flexible form so it was used in the production of plastics, packaging material, insulation, foams for cushioning, and things like the soles of your shoes. CFC-113 has such a low flammability and low toxicity that it was also used as a cleaner for delicate electrical equipment, fabrics, and even metals. Because it would not warm the product it was cleaning, catch fire with a spark or react to and other chemicals it was ideal for this purpose. CFC-113 in laboratory analytics has been replaced by other solvents.; Trichlorotrifluoroethane, also called 1,1,2-Trichloro-1,2,2-trifluoroethane or CFC-113 is a chlorofluorocarbon. It has the formula Cl2FC-CClF2. |
|---|
| Contaminant Sources | - Clean Air Act Chemicals
- DEA Chemicals
- EAFUS Chemicals
- FooDB Chemicals
- HPV EPA Chemicals
- STOFF IDENT Compounds
- ToxCast & Tox21 Chemicals
|
|---|
| Contaminant Type | Not Available |
|---|
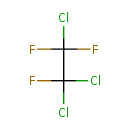
| Chemical Structure | |
|---|
| Synonyms | | Value | Source |
|---|
| 1,1,2-Trichloro-1,2,2-trifluoro-ethane | HMDB | | 1,1,2-Trichloro-1,2,2-trifluoroethane (CFC-113) | HMDB | | 1,1,2-Trichloro-trifluoroethane | HMDB | | 1,1,2-Trichlorotrifluoroethane | HMDB | | 1,1,2-Trichlorotrifluoroethane (freon 113) | HMDB | | 1,1,2-Trifluoro-1,2,2-trichloroethane | HMDB | | 1,1,2-Trifluorotrichloroethane | HMDB | | 1,2,2-Trichlorotrifluoroethane | HMDB | | 1,2,2-Trifluorotrichloroethane | HMDB | | Arcton 113 | HMDB | | Arcton 63 | HMDB | | Arklone | HMDB | | Arklone p | HMDB | | Asahifron 113 | HMDB | | CFC 113 | HMDB | | CFCL2cf2CL | HMDB | | Chlorinated fluorocarbon | HMDB | | Chlorofluorocarbon 113 | HMDB | | Daiflon S 3 | HMDB | | Diflon S-3 | HMDB | | Distillex DS5 | HMDB | | Eskimon 113 | HMDB | | Flugene 113 | HMDB | | Forane | HMDB | | Forane 113 | HMDB | | Freon 113 | HMDB | | Freon 113 TR-T | HMDB | | Freon 113TR-T | HMDB | | Freon F113 | HMDB | | Freon R 113 | HMDB | | Freon TF | HMDB | | Freon TF (113) | HMDB | | Fridohna | HMDB | | Frigen 113 | HMDB | | Frigen 113 TR | HMDB | | Frigen 113 TR-a | HMDB | | Frigen 113 TR-N | HMDB | | Frigen 113 TR-T | HMDB | | Frigen 113a | HMDB | | Frigen 113TR | HMDB | | Frigen 113TR-N | HMDB | | Frigen 113TR-T | HMDB | | Fron 113 | HMDB | | Fronsolve 113 | HMDB | | Genesolv D | HMDB | | Genesolv D sslvent | HMDB | | Genetron 113 | HMDB | | Genetron(R) 113 | HMDB | | Halocarbon 113 | HMDB | | Isceon 113 | HMDB | | Kaiser chemicals 11 | HMDB | | Kaltron 113MDR | HMDB | | Khladon 113 | HMDB | | LEDON 113 | HMDB | | MS-180 Freon TF solv | HMDB | | Propellant 113 | HMDB | | R 113 (Halocarbon) | HMDB | | R 113(Halocarbon) | HMDB | | Racon 113 | HMDB | | Refrigerant 113 | HMDB | | Refrigerant R 113 | HMDB | | Trichloro 1,2,2-trifluoroethane | HMDB | | Trichlorotrifluoroethane | HMDB | | Ucon 113/halocarbon 113 | HMDB | | Ucon fluorocarbon 113 | HMDB |
|
|---|
| Chemical Formula | C2Cl3F3 |
|---|
| Average Molecular Mass | 187.376 g/mol |
|---|
| Monoisotopic Mass | 185.902 g/mol |
|---|
| CAS Registry Number | 76-13-1 |
|---|
| IUPAC Name | 1,1,2-trichloro-1,2,2-trifluoroethane |
|---|
| Traditional Name | 1,1,2-trichlorotrifluoroethane |
|---|
| SMILES | FC(F)(Cl)C(F)(Cl)Cl |
|---|
| InChI Identifier | InChI=1S/C2Cl3F3/c3-1(4,6)2(5,7)8 |
|---|
| InChI Key | AJDIZQLSFPQPEY-UHFFFAOYSA-N |
|---|
| Chemical Taxonomy |
|---|
| Description | belongs to the class of organic compounds known as chlorofluorocarbons. These are alkyhalide compounds that are composed only of chlorine, fluorine, and carbon atoms. |
|---|
| Kingdom | Organic compounds |
|---|
| Super Class | Organohalogen compounds |
|---|
| Class | Alkyl halides |
|---|
| Sub Class | Alkyl chlorides |
|---|
| Direct Parent | Chlorofluorocarbons |
|---|
| Alternative Parents | |
|---|
| Substituents | - Chlorofluorocarbon
- Hydrocarbon derivative
- Organofluoride
- Organochloride
- Alkyl fluoride
- Aliphatic acyclic compound
|
|---|
| Molecular Framework | Aliphatic acyclic compounds |
|---|
| External Descriptors | Not Available |
|---|
| Biological Properties |
|---|
| Status | Detected and Not Quantified |
|---|
| Origin | Not Available |
|---|
| Cellular Locations | Not Available |
|---|
| Biofluid Locations | Not Available |
|---|
| Tissue Locations | Not Available |
|---|
| Pathways | Not Available |
|---|
| Applications | Not Available |
|---|
| Biological Roles | Not Available |
|---|
| Chemical Roles | Not Available |
|---|
| Physical Properties |
|---|
| State | Not Available |
|---|
| Appearance | Not Available |
|---|
| Experimental Properties | | Property | Value |
|---|
| Melting Point | Not Available | | Boiling Point | Not Available | | Solubility | Not Available |
|
|---|
| Predicted Properties | |
|---|
| Spectra |
|---|
| Spectra | | Spectrum Type | Description | Splash Key | View |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | splash10-0uxr-3900000000-df31e868d17737490158 | Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | Not Available | Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | Not Available | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-000i-0900000000-2810e260780354376f67 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-000i-0900000000-2810e260780354376f67 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-000i-0900000000-2810e260780354376f67 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-001i-0900000000-24030ec707f0db03339c | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-001i-0900000000-24030ec707f0db03339c | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-001i-0900000000-24030ec707f0db03339c | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-000i-0900000000-de895e1b22f27fc1d92c | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-000i-0900000000-de895e1b22f27fc1d92c | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-000i-0900000000-247c766c4ae36e619022 | Spectrum |
|
|---|
| Toxicity Profile |
|---|
| Route of Exposure | Not Available |
|---|
| Mechanism of Toxicity | Not Available |
|---|
| Metabolism | Not Available |
|---|
| Toxicity Values | Not Available |
|---|
| Lethal Dose | Not Available |
|---|
| Carcinogenicity (IARC Classification) | Not Available |
|---|
| Uses/Sources | Not Available |
|---|
| Minimum Risk Level | Not Available |
|---|
| Health Effects | Not Available |
|---|
| Symptoms | Not Available |
|---|
| Treatment | Not Available |
|---|
| Concentrations |
|---|
| Not Available |
|---|
| External Links |
|---|
| DrugBank ID | Not Available |
|---|
| HMDB ID | HMDB0031155 |
|---|
| FooDB ID | FDB003169 |
|---|
| Phenol Explorer ID | Not Available |
|---|
| KNApSAcK ID | Not Available |
|---|
| BiGG ID | Not Available |
|---|
| BioCyc ID | Not Available |
|---|
| METLIN ID | Not Available |
|---|
| PDB ID | Not Available |
|---|
| Wikipedia Link | 1,1,2-Trichloro-1,2,2-trifluoroethane |
|---|
| Chemspider ID | 6188 |
|---|
| ChEBI ID | 598103 |
|---|
| PubChem Compound ID | 6428 |
|---|
| Kegg Compound ID | Not Available |
|---|
| YMDB ID | Not Available |
|---|
| ECMDB ID | Not Available |
|---|
| References |
|---|
| Synthesis Reference | Not Available |
|---|
| MSDS | Not Available |
|---|
| General References | |
|---|