| Record Information |
|---|
| Version | 1.0 |
|---|
| Creation Date | 2016-05-19 01:31:22 UTC |
|---|
| Update Date | 2016-10-28 10:00:56 UTC |
|---|
| Accession Number | CHEM003983 |
|---|
| Identification |
|---|
| Common Name | Formic acid |
|---|
| Class | Small Molecule |
|---|
| Description | Formic acid (systematically called methanoic acid) is the simplest carboxylic acid. It is an important intermediate in chemical synthesis and occurs naturally, most famously in the venom of bee and ant stings. It is commonly used as a preservative and antibacterial agent in livestock feed. |
|---|
| Contaminant Sources | - Clean Air Act Chemicals
- DEA Chemicals
- EAFUS Chemicals
- FooDB Chemicals
- HMDB Contaminants - Feces
- HMDB Contaminants - Urine
- HPV EPA Chemicals
- OECD HPV Chemicals
- STOFF IDENT Compounds
- Tobacco Smoke Compounds
|
|---|
| Contaminant Type | Not Available |
|---|
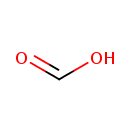
| Chemical Structure | |
|---|
| Synonyms | | Value | Source |
|---|
| Acide formique | ChEBI | | Ameisensaeure | ChEBI | | Aminic acid | ChEBI | | Bilorin | ChEBI | | Formylic acid | ChEBI | | H-COOH | ChEBI | | HCO2H | ChEBI | | HCOOH | ChEBI | | Hydrogen carboxylic acid | ChEBI | | Methanoic acid | ChEBI | | Methoic acid | ChEBI | | Aminate | Generator | | Formylate | Generator | | Hydrogen carboxylate | Generator | | Methanoate | Generator | | Methoate | Generator | | Formate | Generator | | Add-F | HMDB | | Ameisensaure | HMDB | | Collo-bueglatt | HMDB | | Collo-didax | HMDB | | Formira | HMDB | | Formisoton | HMDB | | Methanoic acid monomer | HMDB | | Myrmicyl | HMDB | | Sodium formate | HMDB | | Sybest | HMDB | | Wonderbond hardener m 600l | HMDB | | Calcium formate | HMDB | | Cobalt(II) formate dihydrate | HMDB | | Formic acid, aluminum salt | HMDB | | Formic acid, copper salt | HMDB | | Formic acid, cromium (+3) salt | HMDB | | Lithium formate | HMDB | | Ammonium formate | HMDB | | Formic acid, ammonium (4:1) salt | HMDB | | Formic acid, ammonium salt | HMDB | | Formic acid, calcium salt | HMDB | | Formic acid, copper (+2) salt | HMDB | | Formic acid, lead (+2) salt | HMDB | | Formic acid, lead salt | HMDB | | Formic acid, nickel salt | HMDB | | Formic acid, potassium salt | HMDB | | Formic acid, strontium salt | HMDB | | Mafusol | HMDB | | Ammonium tetraformate | HMDB | | Formic acid, 14C-labeled | HMDB | | Formic acid, cobalt (+2) salt | HMDB | | Formic acid, copper, ammonium salt | HMDB | | Formic acid, sodium salt | HMDB | | Formic acid, sodium salt, 14C-labeled | HMDB | | Formic acid, ammonium (2:1) salt | HMDB | | Formic acid, cadmium salt | HMDB | | Formic acid, cesium salt | HMDB | | Formic acid, copper, nickel salt | HMDB | | Formic acid, cromium (+3), sodium (4:1:1) salt | HMDB | | Formic acid, lithium salt | HMDB | | Formic acid, magnesium salt | HMDB | | Formic acid, nickel (+2) salt | HMDB | | Formic acid, rubidium salt | HMDB | | Formic acid, sodium salt, 13C-labeled | HMDB | | Formic acid, thallium (+1) salt | HMDB | | Formic acid, zinc salt | HMDB | | Nickel formate dihydrate | HMDB | | Aluminum formate | HMDB | | Potassium formate | HMDB | | Strontium formate | HMDB | | Lead formate | HMDB | | Nickel formate | HMDB | | Chromic formate | HMDB | | Cobaltous formate | HMDB | | Cupric formate | HMDB | | Magnesium formate | HMDB | | Zinc formate | HMDB |
|
|---|
| Chemical Formula | CH2O2 |
|---|
| Average Molecular Mass | 46.025 g/mol |
|---|
| Monoisotopic Mass | 46.005 g/mol |
|---|
| CAS Registry Number | 64-18-6 |
|---|
| IUPAC Name | formic acid |
|---|
| Traditional Name | formic acid |
|---|
| SMILES | OC=O |
|---|
| InChI Identifier | InChI=1S/CH2O2/c2-1-3/h1H,(H,2,3) |
|---|
| InChI Key | BDAGIHXWWSANSR-UHFFFAOYSA-N |
|---|
| Chemical Taxonomy |
|---|
| Description | belongs to the class of organic compounds known as carboxylic acids. Carboxylic acids are compounds containing a carboxylic acid group with the formula -C(=O)OH. |
|---|
| Kingdom | Organic compounds |
|---|
| Super Class | Organic acids and derivatives |
|---|
| Class | Carboxylic acids and derivatives |
|---|
| Sub Class | Carboxylic acids |
|---|
| Direct Parent | Carboxylic acids |
|---|
| Alternative Parents | |
|---|
| Substituents | - Monocarboxylic acid or derivatives
- Carboxylic acid
- Organic oxygen compound
- Organic oxide
- Hydrocarbon derivative
- Organooxygen compound
- Carbonyl group
- Aliphatic acyclic compound
|
|---|
| Molecular Framework | Aliphatic acyclic compounds |
|---|
| External Descriptors | |
|---|
| Biological Properties |
|---|
| Status | Detected and Not Quantified |
|---|
| Origin | Not Available |
|---|
| Cellular Locations | Not Available |
|---|
| Biofluid Locations | Not Available |
|---|
| Tissue Locations | Not Available |
|---|
| Pathways | Not Available |
|---|
| Applications | Not Available |
|---|
| Biological Roles | Not Available |
|---|
| Chemical Roles | Not Available |
|---|
| Physical Properties |
|---|
| State | Not Available |
|---|
| Appearance | Not Available |
|---|
| Experimental Properties | | Property | Value |
|---|
| Melting Point | Not Available | | Boiling Point | Not Available | | Solubility | Not Available |
|
|---|
| Predicted Properties | |
|---|
| Spectra |
|---|
| Spectra | | Spectrum Type | Description | Splash Key | View |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | splash10-0002-9000000000-5d27bb312e37a2c8994f | Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (1 TMS) - 70eV, Positive | splash10-0fmi-9200000000-2a89ba98485194acd75a | Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | Not Available | Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (TBDMS_1_1) - 70eV, Positive | Not Available | Spectrum | | LC-MS/MS | LC-MS/MS Spectrum - QqQ 4V, positive | splash10-0002-9000000000-a8fbddf8ca4197b30013 | Spectrum | | LC-MS/MS | LC-MS/MS Spectrum - QqQ 5V, positive | splash10-0002-9000000000-98310116f8a2d6a969ce | Spectrum | | LC-MS/MS | LC-MS/MS Spectrum - QqQ 6V, positive | splash10-0002-9000000000-ec8753fd9790a3cf0be8 | Spectrum | | LC-MS/MS | LC-MS/MS Spectrum - QqQ 7V, positive | splash10-0002-9000000000-121d1a025b72a70e412a | Spectrum | | LC-MS/MS | LC-MS/MS Spectrum - QqQ 8V, positive | splash10-0002-9000000000-5f1955dee7ab988e86dd | Spectrum | | LC-MS/MS | LC-MS/MS Spectrum - QqQ 9V, positive | splash10-0002-9000000000-f8e14272296ee06d19f4 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-0002-9000000000-092f816e62c8d2f5d56e | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-0002-9000000000-092f816e62c8d2f5d56e | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-0002-9000000000-092f816e62c8d2f5d56e | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-0006-9000000000-eb2207f7400e9144fff7 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-0006-9000000000-eb2207f7400e9144fff7 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-0006-9000000000-eb2207f7400e9144fff7 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-0006-9000000000-d948d5d95ae14e701f57 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-0006-9000000000-d948d5d95ae14e701f57 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-0006-9000000000-d948d5d95ae14e701f57 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-0002-9000000000-92190863fc6ae28a8789 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-0002-9000000000-92190863fc6ae28a8789 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-0002-9000000000-348f481062f48991a0aa | Spectrum | | MS | Mass Spectrum (Electron Ionization) | splash10-004j-9000000000-2e63b0c1e2e417b0d747 | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 2D NMR | [1H,1H] 2D NMR Spectrum | Not Available | Spectrum | | 2D NMR | [1H,13C] 2D NMR Spectrum | Not Available | Spectrum |
|
|---|
| Toxicity Profile |
|---|
| Route of Exposure | Not Available |
|---|
| Mechanism of Toxicity | Not Available |
|---|
| Metabolism | Not Available |
|---|
| Toxicity Values | Not Available |
|---|
| Lethal Dose | Not Available |
|---|
| Carcinogenicity (IARC Classification) | Not Available |
|---|
| Uses/Sources | Not Available |
|---|
| Minimum Risk Level | Not Available |
|---|
| Health Effects | Not Available |
|---|
| Symptoms | Not Available |
|---|
| Treatment | Not Available |
|---|
| Concentrations |
|---|
| Not Available |
|---|
| External Links |
|---|
| DrugBank ID | DB01942 |
|---|
| HMDB ID | HMDB0000142 |
|---|
| FooDB ID | FDB012804 |
|---|
| Phenol Explorer ID | Not Available |
|---|
| KNApSAcK ID | C00001182 |
|---|
| BiGG ID | Not Available |
|---|
| BioCyc ID | FORMATE |
|---|
| METLIN ID | Not Available |
|---|
| PDB ID | Not Available |
|---|
| Wikipedia Link | Formic_acid |
|---|
| Chemspider ID | Not Available |
|---|
| ChEBI ID | 30751 |
|---|
| PubChem Compound ID | 284 |
|---|
| Kegg Compound ID | C00058 |
|---|
| YMDB ID | YMDB00385 |
|---|
| ECMDB ID | ECMDB00142 |
|---|
| References |
|---|
| Synthesis Reference | Not Available |
|---|
| MSDS | Not Available |
|---|
| General References | | 1. Finholt, Albert E.; Jacobson, Eugene C. The reduction of carbon dioxide to formic acid with lithium aluminum hydride. Journal of the American Chemical Society (1952), 74 3943-4. | | 2. Sundekilde UK, Gustavsson F, Poulsen NA, Glantz M, Paulsson M, Larsen LB, Bertram HC: Association between the bovine milk metabolome and rennet-induced coagulation properties of milk. J Dairy Sci. 2014 Oct;97(10):6076-84. doi: 10.3168/jds.2014-8304. Epub 2014 Jul 30. | | 3. O'Callaghan TF, Vazquez-Fresno R, Serra-Cayuela A, Dong E, Mandal R, Hennessy D, McAuliffe S, Dillon P, Wishart DS, Stanton C, Ross RP: Pasture Feeding Changes the Bovine Rumen and Milk Metabolome. Metabolites. 2018 Apr 6;8(2). pii: metabo8020027. doi: 10.3390/metabo8020027. | | 4. A. Foroutan et al. The Chemical Composition of Commercial Cow's Milk (in preparation) | | 5. Finholt, Albert E.; Jacobson, Eugene C. The reduction of carbon dioxide to formic acid with lithium aluminum hydride. Journal of the American Chemical Society (1952), 74 3943-4. | | 6. Ohmori S, Sumii I, Toyonaga Y, Nakata K, Kawase M: High-performance liquid chromatographic determination of formate as benzimidazole in biological samples. J Chromatogr. 1988 Apr 8;426(1):15-24. | | 7. Dal Pra I, Chiarini A, Boschi A, Freddi G, Armato U: Novel dermo-epidermal equivalents on silk fibroin-based formic acid-crosslinked three-dimensional nonwoven devices with prospective applications in human tissue engineering/regeneration/repair. Int J Mol Med. 2006 Aug;18(2):241-7. | | 8. Igeta Y, Kawarabayashi T, Sato M, Yamada N, Matsubara E, Ishiguro K, Kanai M, Tomidokoro Y, Osuga J, Okamoto K, Hirai S, Shoji M: Apolipoprotein E accumulates with the progression of A beta deposition in transgenic mice. J Neuropathol Exp Neurol. 1997 Nov;56(11):1228-35. | | 9. Kerns W 2nd, Tomaszewski C, McMartin K, Ford M, Brent J: Formate kinetics in methanol poisoning. J Toxicol Clin Toxicol. 2002;40(2):137-43. | | 10. Nagasawa H, Wada M, Koyama S, Kawanami T, Kurita K, Kato T: [A case of methanol intoxication with optic neuropathy visualized on STIR sequence of MR images]. Rinsho Shinkeigaku. 2005 Jul;45(7):527-30. | | 11. Foulon V, Sniekers M, Huysmans E, Asselberghs S, Mahieu V, Mannaerts GP, Van Veldhoven PP, Casteels M: Breakdown of 2-hydroxylated straight chain fatty acids via peroxisomal 2-hydroxyphytanoyl-CoA lyase: a revised pathway for the alpha-oxidation of straight chain fatty acids. J Biol Chem. 2005 Mar 18;280(11):9802-12. Epub 2005 Jan 11. | | 12. Iwamoto N, Nishiyama E, Ohwada J, Arai H: Distribution of amyloid deposits in the cerebral white matter of the Alzheimer's disease brain: relationship to blood vessels. Acta Neuropathol. 1997 Apr;93(4):334-40. | | 13. Ferrari LA, Arado MG, Nardo CA, Giannuzzi L: Post-mortem analysis of formic acid disposition in acute methanol intoxication. Forensic Sci Int. 2003 Apr 23;133(1-2):152-8. | | 14. Tasaka Y, Nakaya F, Matsumoto H, Iwamoto Y, Omori Y: Pancreatic amylin content in human diabetic subjects and its relation to diabetes. Pancreas. 1995 Oct;11(3):303-8. | | 15. D'Andrea MR, Reiser PA, Polkovitch DA, Gumula NA, Branchide B, Hertzog BM, Schmidheiser D, Belkowski S, Gastard MC, Andrade-Gordon P: The use of formic acid to embellish amyloid plaque detection in Alzheimer's disease tissues misguides key observations. Neurosci Lett. 2003 May 15;342(1-2):114-8. | | 16. Nicholson JK, Foxall PJ, Spraul M, Farrant RD, Lindon JC: 750 MHz 1H and 1H-13C NMR spectroscopy of human blood plasma. Anal Chem. 1995 Mar 1;67(5):793-811. | | 17. Berode M, Sethre T, Laubli T, Savolainen H: Urinary methanol and formic acid as indicators of occupational exposure to methyl formate. Int Arch Occup Environ Health. 2000 Aug;73(6):410-4. | | 18. Lehmann P, Kligman AM: In vivo removal of the horny layer with formic acid. Br J Dermatol. 1983 Sep;109(3):313-20. | | 19. Bales JR, Higham DP, Howe I, Nicholson JK, Sadler PJ: Use of high-resolution proton nuclear magnetic resonance spectroscopy for rapid multi-component analysis of urine. Clin Chem. 1984 Mar;30(3):426-32. | | 20. Dunne VG, Bhattachayya S, Besser M, Rae C, Griffin JL: Metabolites from cerebrospinal fluid in aneurysmal subarachnoid haemorrhage correlate with vasospasm and clinical outcome: a pattern-recognition 1H NMR study. NMR Biomed. 2005 Feb;18(1):24-33. | | 21. Bloomer JC, Clarke SE, Chenery RJ: Determination of P4501A2 activity in human liver microsomes using [3-14C-methyl]caffeine. Xenobiotica. 1995 Sep;25(9):917-27. | | 22. Grady S, Osterloh J: Improved enzymic assay for serum formate with colorimetric endpoint. J Anal Toxicol. 1986 Jan-Feb;10(1):1-5. | | 23. Baumann K, Angerer J: Occupational chronic exposure to organic solvents. VI. Formic acid concentration in blood and urine as an indicator of methanol exposure. Int Arch Occup Environ Health. 1979 Jan 15;42(3-4):241-9. | | 24. Ferry DG, Temple WA, McQueen EG: Methanol monitoring. Comparison of urinary methanol concentration with formic acid excretion rate as a measure of occupational exposure. Int Arch Occup Environ Health. 1980;47(2):155-63. | | 25. Gupta A, Dwivedi M, Mahdi AA, Khetrapal CL, Bhandari M: Broad identification of bacterial type in urinary tract infection using (1)h NMR spectroscopy. J Proteome Res. 2012 Mar 2;11(3):1844-54. doi: 10.1021/pr2010692. Epub 2012 Jan 31. | | 26. https://www.ncbi.nlm.nih.gov/pubmed/?term=12591956 | | 27. https://www.ncbi.nlm.nih.gov/pubmed/?term=14637377 | | 28. https://www.ncbi.nlm.nih.gov/pubmed/?term=15811469 | | 29. https://www.ncbi.nlm.nih.gov/pubmed/?term=16120414 | | 30. https://www.ncbi.nlm.nih.gov/pubmed/?term=16185830 | | 31. https://www.ncbi.nlm.nih.gov/pubmed/?term=16222862 | | 32. https://www.ncbi.nlm.nih.gov/pubmed/?term=16230297 | | 33. https://www.ncbi.nlm.nih.gov/pubmed/?term=16445901 | | 34. https://www.ncbi.nlm.nih.gov/pubmed/?term=16465784 | | 35. https://www.ncbi.nlm.nih.gov/pubmed/?term=18034701 | | 36. https://www.ncbi.nlm.nih.gov/pubmed/?term=18397576 | | 37. https://www.ncbi.nlm.nih.gov/pubmed/?term=22080171 | | 38. https://www.ncbi.nlm.nih.gov/pubmed/?term=22280475 | | 39. https://www.ncbi.nlm.nih.gov/pubmed/?term=22304812 | | 40. https://www.ncbi.nlm.nih.gov/pubmed/?term=22385261 | | 41. https://www.ncbi.nlm.nih.gov/pubmed/?term=22447125 | | 42. https://www.ncbi.nlm.nih.gov/pubmed/?term=22483350 | | 43. https://www.ncbi.nlm.nih.gov/pubmed/?term=22499553 | | 44. https://www.ncbi.nlm.nih.gov/pubmed/?term=22540994 | | 45. https://www.ncbi.nlm.nih.gov/pubmed/?term=22606986 | | 46. https://www.ncbi.nlm.nih.gov/pubmed/?term=22622393 | | 47. https://www.ncbi.nlm.nih.gov/pubmed/?term=3946945 | | 48. https://www.ncbi.nlm.nih.gov/pubmed/?term=7361809 |
|
|---|