| Record Information |
|---|
| Version | 1.0 |
|---|
| Creation Date | 2014-10-02 22:26:52 UTC |
|---|
| Update Date | 2016-11-09 01:09:15 UTC |
|---|
| Accession Number | CHEM003926 |
|---|
| Identification |
|---|
| Common Name | Sodium hypochlorite |
|---|
| Class | Small Molecule |
|---|
| Description | Sodium Hypochlorite is the sodium salt of hypochlorous acid. When dissolved in water it is commonly known as bleach or liquid bleach, and is frequently used as a disinfectant or a bleaching agent. Household bleach is, typically a solution containing 3-8% sodium hypochlorite and 0.01-0.05% sodium hydroxide. The sodium hydroxide is used to slow the decomposition of sodium hypochlorite into sodium chloride and sodium chlorate. In household form, sodium hypochlorite is used for removal of stains from laundry. It is particularly effective on cotton fiber, which stains easily but bleaches well. Most sodium hyphchlorite is sold in aqueous solutions of varying concentrations. |
|---|
| Contaminant Sources | - Clean Air Act Chemicals
- EAFUS Chemicals
- FooDB Chemicals
- HPV EPA Chemicals
- IARC Carcinogens Group 3
- OECD HPV Chemicals
- T3DB toxins
- ToxCast & Tox21 Chemicals
|
|---|
| Contaminant Type | - Disinfectant
- Industrial/Workplace Toxin
- Lachrymator
- Synthetic Compound
|
|---|
| Chemical Structure | |
|---|
| Synonyms | | Value | Source |
|---|
| Hypochlorite sodium | ChEBI | | Hypochlorous acid, sodium salt | ChEBI | | NaClO | ChEBI | | NaOCl | ChEBI | | Sodium oxychloride | ChEBI | | Texant | ChEBI | | Clorox | MeSH | | Hypochlorite, sodium | MeSH | | Sodium hypochlorite (solution) | MeSH | | Antiformin | MeSH |
|
|---|
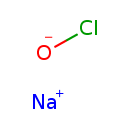
| Chemical Formula | ClNaO |
|---|
| Average Molecular Mass | 74.442 g/mol |
|---|
| Monoisotopic Mass | 73.954 g/mol |
|---|
| CAS Registry Number | 7681-52-9 |
|---|
| IUPAC Name | sodium hypochlorite |
|---|
| Traditional Name | sodium hypochlorite |
|---|
| SMILES | [Na+].[O-]Cl |
|---|
| InChI Identifier | InChI=1S/ClO.Na/c1-2;/q-1;+1 |
|---|
| InChI Key | SUKJFIGYRHOWBL-UHFFFAOYSA-N |
|---|
| Chemical Taxonomy |
|---|
| Description | belongs to the class of inorganic compounds known as alkali metal hypochlorites. These are inorganic compounds in which the largest oxoanion is hypochlorite, and in which the heaviest atom not in an oxoanion is an alkali metal. |
|---|
| Kingdom | Inorganic compounds |
|---|
| Super Class | Mixed metal/non-metal compounds |
|---|
| Class | Alkali metal oxoanionic compounds |
|---|
| Sub Class | Alkali metal hypochlorites |
|---|
| Direct Parent | Alkali metal hypochlorites |
|---|
| Alternative Parents | |
|---|
| Substituents | - Alkali metal hypochlorite
- Inorganic sodium salt
- Inorganic oxide
- Inorganic salt
|
|---|
| Molecular Framework | Not Available |
|---|
| External Descriptors | |
|---|
| Biological Properties |
|---|
| Status | Detected and Not Quantified |
|---|
| Origin | Exogenous |
|---|
| Cellular Locations | |
|---|
| Biofluid Locations | Not Available |
|---|
| Tissue Locations | Not Available |
|---|
| Pathways | | Name | SMPDB Link | KEGG Link |
|---|
| Penicillins | Not Available | Not Available | | Antiviral Agents | Not Available | Not Available | | Fatty acid Metabolism | SMP00051 | map00071 | | Apoptosis | Not Available | map04210 | | Antifungal Agents | Not Available | Not Available | | Sulfur metabolism | Not Available | map00920 | | Rna polymerase | Not Available | map03020 | | Oxidative phosphorylation | Not Available | map00190 |
|
|---|
| Applications | Not Available |
|---|
| Biological Roles | Not Available |
|---|
| Chemical Roles | Not Available |
|---|
| Physical Properties |
|---|
| State | Solid |
|---|
| Appearance | Greenish-yellow solid (pentahydrate) or yellow liquid (as bleach). |
|---|
| Experimental Properties | | Property | Value |
|---|
| Melting Point | 18 °C | | Boiling Point | 101 °C | | Solubility | 29.3 g/100mL |
|
|---|
| Predicted Properties | |
|---|
| Spectra |
|---|
| Spectra | | Spectrum Type | Description | Splash Key | View |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-00di-9000000000-af546d0aa1567991a144 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-00di-9000000000-af546d0aa1567991a144 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-00di-9000000000-2f23d1a7b4470cb8289b | Spectrum |
|
|---|
| Toxicity Profile |
|---|
| Route of Exposure | Dermal |
|---|
| Mechanism of Toxicity | Sodium hypochlorite is a strong oxidizer. Oxidation reactions are corrosive, solutions burn skin and cause eye damage, especially when used in concentrated forms. In particular, hypochorite (HOCl) is known to cause post-translational modifications to amino acids in proteins, the notable ones being cysteine and methionine oxidation. These oxidation reactions can lead to widespread protein aggregation and denaturation, leading to cell death and tissue damage. It is estimated that there are about 3300 accidents needing hospital treatment caused by sodium hypochlorite solutions each year in British homes. A recent European study indicated that sodium hypochlorite and organic chemicals (e.g., surfactants, fragrances) contained in several household cleaning products can react to generate chlorinated volatile organic compounds (VOCs). Some of these VOCs are toxic and probable human carcinogens. The study showed that indoor air concentrations significantly increase (8-52 times for chloroform and 1-1170 times for carbon tetrachloride, respectively, above baseline quantities in the household) during the use of bleach containing products. Sodium Hypochlorite reacts violently with amines and ammonium salts. Solutions are reactive with common cleaning products such as toilet bowl cleaners, rust removers, vinegar, acids, organics and ammonia products to produce hazardous gases such as chlorine and other chlorinated species. |
|---|
| Metabolism | Not Available |
|---|
| Toxicity Values | Not Available |
|---|
| Lethal Dose | Not Available |
|---|
| Carcinogenicity (IARC Classification) | 3, not classifiable as to its carcinogenicity to humans. (1) |
|---|
| Uses/Sources | Sodium hypochlorite is frequently used as a disinfectant or a bleaching agent.
|
|---|
| Minimum Risk Level | Not Available |
|---|
| Health Effects | Chlorine Bleach is a strong corrosive material. Sodium hypochlorite solutions frequently produce small amounts of chlorine gas. If inhaled, chlorine can trigger cough, substernal pain, respiratory distress, shortness of breath, and wheezing. Symptoms may be delayed. Nausea and vomiting are reflex in origin, and headache and loss of consciousness are probably due to the hypoxia caused by pulmonary edema. Dermal contact can lead to redness, pain, and redness of the exposed surface. Eye contact can lead to watering of the eyes. Ingestion can cause pulmonary edema, vomiting or coma. Exposure to the skin may cause sensitization or other allergic responses. If the eye is not irrigated immediately after it has been exposed permanent eye damage may occur. Toxicity described in animals from single low-dose (1% solution) exposures by ingestion include muscular weakness, and hypoactivity. Long-term administration of compound in drinking water of rats caused depression of the immune system. No adverse changes were observed in an eight week dermal study of a 1% solution in guinea pigs. Tests in animals demonstrate no carcinogenic activity by either the oral or dermal routes. |
|---|
| Symptoms | Not Available |
|---|
| Treatment | EYES: irrigate opened eyes for several minutes under running water. INGESTION: do not induce vomiting. Rinse mouth with water (never give anything by mouth to an unconscious person). Seek immediate medical advice. SKIN: should be treated immediately by rinsing the affected parts in cold running water for at least 15 minutes, followed by thorough washing with soap and water. If necessary, the person should shower and change contaminated clothing and shoes, and then must seek medical attention. INHALATION: supply fresh air. If required provide artificial respiration. |
|---|
| Concentrations |
|---|
| Not Available |
|---|
| External Links |
|---|
| DrugBank ID | DBSALT001517 |
|---|
| HMDB ID | HMDB0303519 |
|---|
| FooDB ID | FDB015395 |
|---|
| Phenol Explorer ID | Not Available |
|---|
| KNApSAcK ID | Not Available |
|---|
| BiGG ID | Not Available |
|---|
| BioCyc ID | Not Available |
|---|
| METLIN ID | Not Available |
|---|
| PDB ID | Not Available |
|---|
| Wikipedia Link | Sodium_hypochlorite |
|---|
| Chemspider ID | 22756 |
|---|
| ChEBI ID | 32146 |
|---|
| PubChem Compound ID | Not Available |
|---|
| Kegg Compound ID | Not Available |
|---|
| YMDB ID | Not Available |
|---|
| ECMDB ID | Not Available |
|---|
| References |
|---|
| Synthesis Reference | Not Available |
|---|
| MSDS | Not Available |
|---|
| General References | |
|---|