| Record Information |
|---|
| Version | 1.0 |
|---|
| Creation Date | 2014-09-25 19:13:14 UTC |
|---|
| Update Date | 2016-11-09 01:09:14 UTC |
|---|
| Accession Number | CHEM003912 |
|---|
| Identification |
|---|
| Common Name | Tritium |
|---|
| Class | Small Molecule |
|---|
| Description | Tritium (also known as hydrogen-3) is a radioactive isotope of hydrogen. The nucleus of tritium (sometimes called a triton) contains one proton and two neutrons, whereas the nucleus of protium (by far the most abundant hydrogen isotope) contains one proton and no neutrons. Naturally occurring tritium is extremely rare on Earth, where trace amounts are formed by the interaction of the atmosphere with cosmic rays. The name of this isotope is formed from the Greek word "tritos" meaning "third". |
|---|
| Contaminant Sources | - IARC Carcinogens Group 1
- T3DB toxins
|
|---|
| Contaminant Type | - Natural Compound
- Pollutant
- Radioactive
|
|---|
| Chemical Structure | |
|---|
| Synonyms | | Value | Source |
|---|
| (3)H2 | ChEBI | | T2 | ChEBI | | Hydrogen-3 | MeSH | | Hydrogen 3 | MeSH |
|
|---|
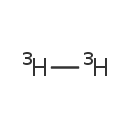
| Chemical Formula | H2 |
|---|
| Average Molecular Mass | 6.032 g/mol |
|---|
| Monoisotopic Mass | 6.032 g/mol |
|---|
| CAS Registry Number | 10028-17-8 |
|---|
| IUPAC Name | (³H₂)dihydrogen |
|---|
| Traditional Name | tritium |
|---|
| SMILES | [3H][3H] |
|---|
| InChI Identifier | InChI=1S/H2/h1H/i1+2T |
|---|
| InChI Key | UFHFLCQGNIYNRP-JMRXTUGHSA-N |
|---|
| Chemical Taxonomy |
|---|
| Description | belongs to the class of inorganic compounds known as other non-metal hydrides. These are inorganic compounds in which the heaviest atom bonded to a hydrogen atom is belongs to the class of 'other non-metals'. |
|---|
| Kingdom | Inorganic compounds |
|---|
| Super Class | Homogeneous non-metal compounds |
|---|
| Class | Other non-metal organides |
|---|
| Sub Class | Other non-metal hydrides |
|---|
| Direct Parent | Other non-metal hydrides |
|---|
| Alternative Parents | Not Available |
|---|
| Substituents | |
|---|
| Molecular Framework | Not Available |
|---|
| External Descriptors | |
|---|
| Biological Properties |
|---|
| Status | Detected and Not Quantified |
|---|
| Origin | Exogenous |
|---|
| Cellular Locations | - Cell surface
- Endoplasmic reticulum
- Extracellular
- Mitochondrion
- Nucleolus
- Nucleoplasm
- Plasma Membrane
- Ribosome
- Synaptic Vesicle
|
|---|
| Biofluid Locations | Not Available |
|---|
| Tissue Locations | Not Available |
|---|
| Pathways | | Name | SMPDB Link | KEGG Link |
|---|
| Eicosanoids | Not Available | Not Available | | Homologous recombination | Not Available | map03440 | | Oxidative phosphorylation | Not Available | map00190 | | Cell cycle | Not Available | map04110 | | Apoptosis | Not Available | map04210 | | Thyroid hormone synthesis | SMP00716 | Not Available | | Phenothiazines | Not Available | Not Available | | Non-homologous end-joining | Not Available | map03450 | | Dna replication | Not Available | map03030 | | Carbon Metabolism | Not Available | Not Available | | Biosynthesis Of Amino Acids | Not Available | Not Available | | Abc transporters | Not Available | map02010 |
|
|---|
| Applications | Not Available |
|---|
| Biological Roles | Not Available |
|---|
| Chemical Roles | Not Available |
|---|
| Physical Properties |
|---|
| State | Not Available |
|---|
| Appearance | NA |
|---|
| Experimental Properties | | Property | Value |
|---|
| Melting Point | Not Available | | Boiling Point | Not Available | | Solubility | Not Available |
|
|---|
| Predicted Properties | |
|---|
| Spectra |
|---|
| Spectra | | Spectrum Type | Description | Splash Key | View |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-0udi-9000000000-0595c445077141efd7ac | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-0udi-9000000000-0595c445077141efd7ac | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-0udi-9000000000-0595c445077141efd7ac | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-0udi-9000000000-cbcf030cfea82ec88da3 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-0udi-9000000000-cbcf030cfea82ec88da3 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-0udi-9000000000-cbcf030cfea82ec88da3 | Spectrum |
|
|---|
| Toxicity Profile |
|---|
| Route of Exposure | Inhalation; ingestion; dermal |
|---|
| Mechanism of Toxicity | Tritium is an isotope of hydrogen, which allows it to readily bind to hydroxyl radicals, forming tritiated water (HTO), and to carbon atoms. Since tritium is a low energy beta emitter, it is not dangerous externally (its beta particles are unable to penetrate the skin), but it is a radiation hazard when inhaled, ingested via food or water, or absorbed through the skin. Beta particles are able to penetrate living matter to a certain extent and can change the structure of struck molecules. In most cases, such change can be considered to be damage, with results possibly as severe as cancer or death. If the struck molecule is DNA, it can cause spontaneous mutation. (Wikipedia) |
|---|
| Metabolism | Tritiated water (HTO) has a short biological half-life in the human body of 7 to 14 days, which both reduces the total effects of single-incident ingestion and precludes long-term bioaccumulation of HTO from the environment. Biological half life of tritiated water in human body, which is a measure of body water turn over, varies with season. Studies on biological half life of occupational radiation workers for free water tritium in the coastal region of Karnataka, India show that the biological half life in winter season is twice that of the summer season. (Wikipedia) |
|---|
| Toxicity Values | The median lethal dose (LD50) of tritium assimilated by the body is estimated to be 370 GBq (10 Ci). Higher doses can be tolerated with forced fluid intake to reduce the biological half life. (1) |
|---|
| Lethal Dose | Not Available |
|---|
| Carcinogenicity (IARC Classification) | 1, carcinogenic to humans. (2) |
|---|
| Uses/Sources | Tritium occurs naturally due to cosmic rays interacting with atmospheric gases. Tritium is produced in nuclear reactors by neutron activation of lithium-6. Tritium is also produced in heavy water-moderated reactors whenever a deuterium nucleus captures a neutron. Tritium is an uncommon product of the nuclear fission of uranium-235, plutonium-239, and uranium-233, with a production of about one atom per each 10,000 fissions. Tritium is used in self-powered lighting, nuclear weapons, and controlled nuclear fusion. It is also used in analytical chemistry as a radiolabel. (Wikipedia) |
|---|
| Minimum Risk Level | Not Available |
|---|
| Health Effects | Cancer, possibly death. |
|---|
| Symptoms | Not Available |
|---|
| Treatment | Not Available |
|---|
| Concentrations |
|---|
| Not Available |
|---|
| External Links |
|---|
| DrugBank ID | Not Available |
|---|
| HMDB ID | Not Available |
|---|
| FooDB ID | Not Available |
|---|
| Phenol Explorer ID | Not Available |
|---|
| KNApSAcK ID | Not Available |
|---|
| BiGG ID | Not Available |
|---|
| BioCyc ID | Not Available |
|---|
| METLIN ID | Not Available |
|---|
| PDB ID | Not Available |
|---|
| Wikipedia Link | Tritium |
|---|
| Chemspider ID | Not Available |
|---|
| ChEBI ID | 29298 |
|---|
| PubChem Compound ID | 24824 |
|---|
| Kegg Compound ID | Not Available |
|---|
| YMDB ID | Not Available |
|---|
| ECMDB ID | Not Available |
|---|
| References |
|---|
| Synthesis Reference | Not Available |
|---|
| MSDS | Not Available |
|---|
| General References | Not Available |
|---|