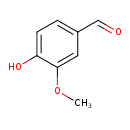
| GC-MS | GC-MS Spectrum - EI-B (Non-derivatized) | splash10-0udi-1900000000-46e0edb3260b8896145e | Spectrum |
| GC-MS | GC-MS Spectrum - EI-B (Non-derivatized) | splash10-0udi-7900000000-ee0a0ad1232fa889e567 | Spectrum |
| GC-MS | GC-MS Spectrum - EI-B (Non-derivatized) | splash10-0udi-9800000000-d8f749f48a78e7c9c3bf | Spectrum |
| GC-MS | GC-MS Spectrum - GC-EI-TOF (Non-derivatized) | splash10-00di-1960000000-b6678c7961ee7df779a1 | Spectrum |
| GC-MS | GC-MS Spectrum - EI-B (Non-derivatized) | splash10-0udi-1900000000-46e0edb3260b8896145e | Spectrum |
| GC-MS | GC-MS Spectrum - EI-B (Non-derivatized) | splash10-0udi-7900000000-ee0a0ad1232fa889e567 | Spectrum |
| GC-MS | GC-MS Spectrum - EI-B (Non-derivatized) | splash10-0udi-9800000000-d8f749f48a78e7c9c3bf | Spectrum |
| GC-MS | GC-MS Spectrum - GC-EI-TOF (Non-derivatized) | splash10-00di-1960000000-b6678c7961ee7df779a1 | Spectrum |
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | splash10-0fk9-1900000000-68ebbe847e88a001f8fa | Spectrum |
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (1 TMS) - 70eV, Positive | splash10-05fr-7950000000-08c9e1cb0dbb3704f8ee | Spectrum |
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | Not Available | Spectrum |
| LC-MS/MS | LC-MS/MS Spectrum - DI-ESI-qTof , Positive | splash10-0udi-0900000000-334e2cf1c60f75229fd1 | Spectrum |
| LC-MS/MS | LC-MS/MS Spectrum - DI-ESI-qTof , Negative | splash10-0uy0-0900200200-4cbd13b45290ded181ee | Spectrum |
| LC-MS/MS | LC-MS/MS Spectrum - LC-ESI-ITFT , negative | splash10-0udr-0900000000-d67b08f1b4f6e90cd270 | Spectrum |
| LC-MS/MS | LC-MS/MS Spectrum - 10V, Negative | splash10-000i-1900000000-9fc5182650ced222b1a1 | Spectrum |
| LC-MS/MS | LC-MS/MS Spectrum - 40V, Negative | splash10-052b-9800000000-312c82e1fbcda8abeaf7 | Spectrum |
| LC-MS/MS | LC-MS/MS Spectrum - 90V, Negative | splash10-0a4j-9700000000-c0da5ed12872161ea30e | Spectrum |
| LC-MS/MS | LC-MS/MS Spectrum - 20V, Negative | splash10-000l-5900000000-8c4d3cd8abfc264729a3 | Spectrum |
| LC-MS/MS | LC-MS/MS Spectrum - 40V, Negative | splash10-00kf-9000000000-a9b7f966d85c30f6174c | Spectrum |
| LC-MS/MS | LC-MS/MS Spectrum - 10V, Negative | splash10-000i-0900000000-d1e812bea83f6f84570d | Spectrum |
| LC-MS/MS | LC-MS/MS Spectrum - 20V, Negative | splash10-000f-7900000000-9d9283afc35603b0121c | Spectrum |
| LC-MS/MS | LC-MS/MS Spectrum - 40V, Positive | splash10-014i-9000000000-146c4b184605c821d822 | Spectrum |
| LC-MS/MS | LC-MS/MS Spectrum - 10V, Positive | splash10-00kf-9500000000-f5a8328127a89c12c19e | Spectrum |
| LC-MS/MS | LC-MS/MS Spectrum - 35V, Positive | splash10-014i-9000000000-44efadd4b0c1fe89c39c | Spectrum |
| LC-MS/MS | LC-MS/MS Spectrum - 20V, Positive | splash10-014i-9100000000-70cac2e87a3ecb4ec867 | Spectrum |
| LC-MS/MS | LC-MS/MS Spectrum - 15V, Negative | splash10-0udr-0900000000-10b0539cab1169d8d877 | Spectrum |
| LC-MS/MS | LC-MS/MS Spectrum - 35V, Negative | splash10-000i-0900000000-17943fa1f4f19dd4878a | Spectrum |
| LC-MS/MS | LC-MS/MS Spectrum - 30V, Negative | splash10-0udr-0900000000-2e50ec52313e18cddbd0 | Spectrum |
| LC-MS/MS | LC-MS/MS Spectrum - 45V, Negative | splash10-0f79-1900000000-98d502286eb47c703f64 | Spectrum |
| LC-MS/MS | LC-MS/MS Spectrum - 75V, Negative | splash10-052e-9800000000-03597a1112a480b91d5e | Spectrum |
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-0udi-0900000000-e949414f752aab5e9606 | Spectrum |
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-0udi-0900000000-1dcee0be3ba8e1431a7c | Spectrum |
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-0ff9-9400000000-dcd0372357bd9c46926d | Spectrum |
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-0udi-0900000000-6cc5e0ed993af0b790e6 | Spectrum |
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-0udi-1900000000-cf1b6af77251971154f9 | Spectrum |
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-000l-9500000000-302ce8740fbb7b9295b9 | Spectrum |
| MS | Mass Spectrum (Electron Ionization) | splash10-0udi-5900000000-907c9684d5ac0386d522 | Spectrum |
| 1D NMR | 1H NMR Spectrum | Not Available | Spectrum |
| 1D NMR | 13C NMR Spectrum | Not Available | Spectrum |
| 1D NMR | 13C NMR Spectrum | Not Available | Spectrum |
| 1D NMR | 1H NMR Spectrum | Not Available | Spectrum |
| 1D NMR | 13C NMR Spectrum | Not Available | Spectrum |
| 1D NMR | 1H NMR Spectrum | Not Available | Spectrum |
| 1D NMR | 13C NMR Spectrum | Not Available | Spectrum |
| 1D NMR | 1H NMR Spectrum | Not Available | Spectrum |
| 1D NMR | 13C NMR Spectrum | Not Available | Spectrum |
| 1D NMR | 1H NMR Spectrum | Not Available | Spectrum |
| 1D NMR | 13C NMR Spectrum | Not Available | Spectrum |
| 1D NMR | 1H NMR Spectrum | Not Available | Spectrum |
| 1D NMR | 13C NMR Spectrum | Not Available | Spectrum |
| 1D NMR | 1H NMR Spectrum | Not Available | Spectrum |
| 1D NMR | 13C NMR Spectrum | Not Available | Spectrum |
| 1D NMR | 1H NMR Spectrum | Not Available | Spectrum |
| 1D NMR | 13C NMR Spectrum | Not Available | Spectrum |
| 1D NMR | 1H NMR Spectrum | Not Available | Spectrum |
| 1D NMR | 13C NMR Spectrum | Not Available | Spectrum |
| 1D NMR | 1H NMR Spectrum | Not Available | Spectrum |
| 1D NMR | 13C NMR Spectrum | Not Available | Spectrum |
| 1D NMR | 1H NMR Spectrum | Not Available | Spectrum |