| Record Information |
|---|
| Version | 1.0 |
|---|
| Creation Date | 2014-09-11 05:18:11 UTC |
|---|
| Update Date | 2016-11-09 01:09:13 UTC |
|---|
| Accession Number | CHEM003783 |
|---|
| Identification |
|---|
| Common Name | Forskolin |
|---|
| Class | Small Molecule |
|---|
| Description | Potent activator of the adenylate cyclase system and the biosynthesis of cyclic AMP. From the plant Coleus forskohlii. Has antihypertensive, positive ionotropic, platelet aggregation inhibitory, and smooth muscle relaxant activities; also lowers intraocular pressure and promotes release of hormones from the pituitary gland. |
|---|
| Contaminant Sources | - T3DB toxins
- ToxCast & Tox21 Chemicals
|
|---|
| Contaminant Type | - Ester
- Ether
- Natural Compound
- Organic Compound
- Plant Toxin
|
|---|
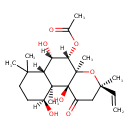
| Chemical Structure | |
|---|
| Synonyms | | Value | Source |
|---|
| 7beta-Acetoxy-8,13-epoxy-1alpha,6beta,9alpha-trihydroxylabd-14-en-11-one | ChEBI | | Coleonol | ChEBI | | Coleonolk | ChEBI | | Colforsina | ChEBI | | Colforsine | ChEBI | | Colforsinum | ChEBI | | Forskolin | Kegg | | 7b-Acetoxy-8,13-epoxy-1a,6b,9a-trihydroxylabd-14-en-11-one | Generator | | 7Β-acetoxy-8,13-epoxy-1α,6β,9α-trihydroxylabd-14-en-11-one | Generator | | Colforsin | ChEBI | | N,N-Dimethyl-beta-alanine-5-(acetyloxy)-3-ethenyldodecahydro-10,10b-dihydroxy-3,4a,7,7,10a-pentamethyl-1-oxo-1H-naphtho(2,1-b)pyran-6-yl ester HCL | MeSH | | NKH 477 | MeSH | | NKH-477 | MeSH |
|
|---|
| Chemical Formula | C22H34O7 |
|---|
| Average Molecular Mass | 410.501 g/mol |
|---|
| Monoisotopic Mass | 410.230 g/mol |
|---|
| CAS Registry Number | 64657-11-0 and 66575-29-9 |
|---|
| IUPAC Name | (3R,4aR,5S,6S,6aS,10S,10aR,10bS)-3-ethenyl-6,10,10b-trihydroxy-3,4a,7,7,10a-pentamethyl-1-oxo-dodecahydro-1H-naphtho[2,1-b]pyran-5-yl acetate |
|---|
| Traditional Name | forskolin |
|---|
| SMILES | [H][C@]1(O)CCC(C)(C)[C@]2([H])[C@]([H])(O)[C@]([H])(OC(C)=O)[C@@]3(C)O[C@](C)(CC(=O)[C@]3(O)[C@@]12C)C=C |
|---|
| InChI Identifier | InChI=1S/C22H34O7/c1-8-19(5)11-14(25)22(27)20(6)13(24)9-10-18(3,4)16(20)15(26)17(28-12(2)23)21(22,7)29-19/h8,13,15-17,24,26-27H,1,9-11H2,2-7H3/t13-,15-,16-,17-,19-,20-,21+,22-/m0/s1 |
|---|
| InChI Key | OHCQJHSOBUTRHG-KGGHGJDLSA-N |
|---|
| Chemical Taxonomy |
|---|
| Description | belongs to the class of organic compounds known as triterpenoids. These are terpene molecules containing six isoprene units. |
|---|
| Kingdom | Organic compounds |
|---|
| Super Class | Lipids and lipid-like molecules |
|---|
| Class | Prenol lipids |
|---|
| Sub Class | Triterpenoids |
|---|
| Direct Parent | Triterpenoids |
|---|
| Alternative Parents | |
|---|
| Substituents | - Polycyclic triterpenoid
- Triterpenoid
- Naphthopyran
- Naphthalene
- Pyran
- Oxane
- Cyclic alcohol
- Tertiary alcohol
- Carboxylic acid ester
- Ketone
- Secondary alcohol
- Carboxylic acid derivative
- Dialkyl ether
- Ether
- Monocarboxylic acid or derivatives
- Oxacycle
- Organoheterocyclic compound
- Polyol
- Carbonyl group
- Hydrocarbon derivative
- Alcohol
- Organic oxygen compound
- Organic oxide
- Organooxygen compound
- Aliphatic heteropolycyclic compound
|
|---|
| Molecular Framework | Aliphatic heteropolycyclic compounds |
|---|
| External Descriptors | |
|---|
| Biological Properties |
|---|
| Status | Detected and Not Quantified |
|---|
| Origin | Exogenous |
|---|
| Cellular Locations | |
|---|
| Biofluid Locations | Not Available |
|---|
| Tissue Locations | Not Available |
|---|
| Pathways | Not Available |
|---|
| Applications | Not Available |
|---|
| Biological Roles | Not Available |
|---|
| Chemical Roles | Not Available |
|---|
| Physical Properties |
|---|
| State | Solid |
|---|
| Appearance | White powder. |
|---|
| Experimental Properties | | Property | Value |
|---|
| Melting Point | Not Available | | Boiling Point | Not Available | | Solubility | Not Available |
|
|---|
| Predicted Properties | |
|---|
| Spectra |
|---|
| Spectra | | Spectrum Type | Description | Splash Key | View |
|---|
| LC-MS/MS | LC-MS/MS Spectrum - LC-ESI-QTOF , negative | splash10-0a4i-0001900000-7d8f5636999f6b276cd0 | Spectrum | | LC-MS/MS | LC-MS/MS Spectrum - LC-ESI-QTOF , negative | splash10-0k92-0349200000-7e9f95896c6366092dc4 | Spectrum | | LC-MS/MS | LC-MS/MS Spectrum - LC-ESI-QTOF , negative | splash10-0zgi-0895000000-d5ffd6e415b7071865cd | Spectrum | | LC-MS/MS | LC-MS/MS Spectrum - LC-ESI-QTOF , negative | splash10-0zfr-0941000000-0d4b697cc719902e9cd2 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-01ox-1009200000-20fab1621372984846a1 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-0uxu-2009000000-f130396db84635f9cd93 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-0gbi-9101000000-237fd86ca77bf89a6101 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-0a4i-3009600000-51bddbda26cd550ea75a | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-0a4j-6139100000-fb3680fca9d4a76753d9 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-0aor-9000000000-894a6ba44b323c93d1b5 | Spectrum |
|
|---|
| Toxicity Profile |
|---|
| Route of Exposure | Not Available |
|---|
| Mechanism of Toxicity | Not Available |
|---|
| Metabolism | Not Available |
|---|
| Toxicity Values | Not Available |
|---|
| Lethal Dose | Not Available |
|---|
| Carcinogenicity (IARC Classification) | No indication of carcinogenicity to humans (not listed by IARC). |
|---|
| Uses/Sources | Not Available |
|---|
| Minimum Risk Level | Not Available |
|---|
| Health Effects | Not Available |
|---|
| Symptoms | Not Available |
|---|
| Treatment | Not Available |
|---|
| Concentrations |
|---|
| Not Available |
|---|
| External Links |
|---|
| DrugBank ID | DB02587 |
|---|
| HMDB ID | Not Available |
|---|
| FooDB ID | Not Available |
|---|
| Phenol Explorer ID | Not Available |
|---|
| KNApSAcK ID | C00003416 |
|---|
| BiGG ID | Not Available |
|---|
| BioCyc ID | Not Available |
|---|
| METLIN ID | Not Available |
|---|
| PDB ID | Not Available |
|---|
| Wikipedia Link | Forskolin |
|---|
| Chemspider ID | Not Available |
|---|
| ChEBI ID | 42471 |
|---|
| PubChem Compound ID | 47936 |
|---|
| Kegg Compound ID | C09076 |
|---|
| YMDB ID | Not Available |
|---|
| ECMDB ID | Not Available |
|---|
| References |
|---|
| Synthesis Reference | Emiko Yamasaki, Takaaki Ohkuma, “Lyophilized preparation of 6-(3-dimethylaminopropionyl)forskolin.” U.S. Patent US5051132, issued June, 1974. |
|---|
| MSDS | Not Available |
|---|
| General References | |
|---|