| Record Information |
|---|
| Version | 1.0 |
|---|
| Creation Date | 2014-09-11 05:17:35 UTC |
|---|
| Update Date | 2016-11-09 01:09:13 UTC |
|---|
| Accession Number | CHEM003767 |
|---|
| Identification |
|---|
| Common Name | N-Butyl-Benzenesulfonamide |
|---|
| Class | Small Molecule |
|---|
| Description | N-Butyl benzenesulfonamide (NBBS), a plasticizer used commercially in the polymerization of polyamide compounds. It is neurotoxic and has been found to induce spastic myelopathy in rabbits. |
|---|
| Contaminant Sources | - HPV EPA Chemicals
- STOFF IDENT Compounds
- T3DB toxins
- ToxCast & Tox21 Chemicals
|
|---|
| Contaminant Type | - Amide
- Industrial/Workplace Toxin
- Organic Compound
- Plasticizer
- Synthetic Compound
|
|---|
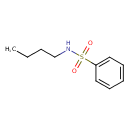
| Chemical Structure | |
|---|
| Synonyms | | Value | Source |
|---|
| Benzenesulfonic acid butyl amide | ChEBI | | Dellatol BBS | ChEBI | | N-(N-Butyl)benzenesulfonamide | ChEBI | | Plastomoll BMB | ChEBI | | Benzenesulfonate butyl amide | Generator | | Benzenesulphonate butyl amide | Generator | | Benzenesulphonic acid butyl amide | Generator | | N-(N-Butyl)benzenesulphonamide | Generator | | N-Butyl-benzenesulphonamide | Generator | | N-Butylbenzenesulphonamide | MeSH | | NBBS | MeSH |
|
|---|
| Chemical Formula | C10H15NO2S |
|---|
| Average Molecular Mass | 213.297 g/mol |
|---|
| Monoisotopic Mass | 213.082 g/mol |
|---|
| CAS Registry Number | 3622-84-2 |
|---|
| IUPAC Name | N-butylbenzenesulfonamide |
|---|
| Traditional Name | N-butyl-benzenesulfonamide |
|---|
| SMILES | CCCCNS(=O)(=O)C1=CC=CC=C1 |
|---|
| InChI Identifier | InChI=1S/C10H15NO2S/c1-2-3-9-11-14(12,13)10-7-5-4-6-8-10/h4-8,11H,2-3,9H2,1H3 |
|---|
| InChI Key | IPRJXAGUEGOFGG-UHFFFAOYSA-N |
|---|
| Chemical Taxonomy |
|---|
| Description | belongs to the class of organic compounds known as benzenesulfonamides. These are organic compounds containing a sulfonamide group that is S-linked to a benzene ring. |
|---|
| Kingdom | Organic compounds |
|---|
| Super Class | Benzenoids |
|---|
| Class | Benzene and substituted derivatives |
|---|
| Sub Class | Benzenesulfonamides |
|---|
| Direct Parent | Benzenesulfonamides |
|---|
| Alternative Parents | |
|---|
| Substituents | - Benzenesulfonamide
- Benzenesulfonyl group
- Organosulfonic acid amide
- Aminosulfonyl compound
- Sulfonyl
- Organosulfonic acid or derivatives
- Organic sulfonic acid or derivatives
- Organic nitrogen compound
- Organic oxygen compound
- Organopnictogen compound
- Organic oxide
- Hydrocarbon derivative
- Organosulfur compound
- Organonitrogen compound
- Aromatic homomonocyclic compound
|
|---|
| Molecular Framework | Aromatic homomonocyclic compounds |
|---|
| External Descriptors | |
|---|
| Biological Properties |
|---|
| Status | Detected and Not Quantified |
|---|
| Origin | Exogenous |
|---|
| Cellular Locations | |
|---|
| Biofluid Locations | Not Available |
|---|
| Tissue Locations | Not Available |
|---|
| Pathways | Not Available |
|---|
| Applications | Not Available |
|---|
| Biological Roles | Not Available |
|---|
| Chemical Roles | Not Available |
|---|
| Physical Properties |
|---|
| State | Solid |
|---|
| Appearance | White powder. |
|---|
| Experimental Properties | | Property | Value |
|---|
| Melting Point | Not Available | | Boiling Point | 314°C | | Solubility | Not Available |
|
|---|
| Predicted Properties | |
|---|
| Spectra |
|---|
| Spectra | | Spectrum Type | Description | Splash Key | View |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | splash10-0006-9600000000-cd5f37e36b479444f0f9 | Spectrum | | LC-MS/MS | LC-MS/MS Spectrum - LC-ESI-ITFT , positive | splash10-0udi-0900000000-ac17d7bd97de41746cb5 | Spectrum | | LC-MS/MS | LC-MS/MS Spectrum - LC-ESI-ITFT , positive | splash10-0udi-0900000000-4b3fba1a84e8a9817ce9 | Spectrum | | LC-MS/MS | LC-MS/MS Spectrum - LC-ESI-ITFT , positive | splash10-0udi-0900000000-0be94919e879316d19cd | Spectrum | | LC-MS/MS | LC-MS/MS Spectrum - LC-ESI-ITFT , positive | splash10-0udi-0900000000-b21e55783effd7b7e375 | Spectrum | | LC-MS/MS | LC-MS/MS Spectrum - LC-ESI-QFT , positive | splash10-052g-5900000000-5d23dc1ef544f9511251 | Spectrum | | LC-MS/MS | LC-MS/MS Spectrum - LC-ESI-QFT , positive | splash10-052f-5900000000-2fa472f57a904ec60c24 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-03di-4390000000-fb042a5e10a6b299aab5 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-0a4i-9200000000-a8348044b7d5917c69a1 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-0a4i-9000000000-5d5130787a19b8df715d | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-03di-0190000000-48cfcfa90253b362d61e | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-01ox-2940000000-174036b1d03d03f8c06a | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-004l-9400000000-c79d7477aa40e59f480b | Spectrum | | MS | Mass Spectrum (Electron Ionization) | splash10-00bc-8900000000-6d3edc2b6cee3acc8852 | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum |
|
|---|
| Toxicity Profile |
|---|
| Route of Exposure | Not Available |
|---|
| Mechanism of Toxicity | Not Available |
|---|
| Metabolism | Not Available |
|---|
| Toxicity Values | Not Available |
|---|
| Lethal Dose | Not Available |
|---|
| Carcinogenicity (IARC Classification) | No indication of carcinogenicity to humans (not listed by IARC). |
|---|
| Uses/Sources | N-Butyl benzenesulfonamide (NBBS), a plasticizer used commercially in the polymerization of polyamide compounds. It is neurotoxic and has been found to induce spastic myelopathy in rabbits. |
|---|
| Minimum Risk Level | Not Available |
|---|
| Health Effects | Not Available |
|---|
| Symptoms | Not Available |
|---|
| Treatment | Not Available |
|---|
| Concentrations |
|---|
| Not Available |
|---|
| External Links |
|---|
| DrugBank ID | DB02055 |
|---|
| HMDB ID | HMDB0062139 |
|---|
| FooDB ID | Not Available |
|---|
| Phenol Explorer ID | Not Available |
|---|
| KNApSAcK ID | Not Available |
|---|
| BiGG ID | Not Available |
|---|
| BioCyc ID | Not Available |
|---|
| METLIN ID | Not Available |
|---|
| PDB ID | Not Available |
|---|
| Wikipedia Link | Not Available |
|---|
| Chemspider ID | Not Available |
|---|
| ChEBI ID | 44237 |
|---|
| PubChem Compound ID | 19241 |
|---|
| Kegg Compound ID | Not Available |
|---|
| YMDB ID | Not Available |
|---|
| ECMDB ID | Not Available |
|---|
| References |
|---|
| Synthesis Reference | Not Available |
|---|
| MSDS | Not Available |
|---|
| General References | |
|---|