| Record Information |
|---|
| Version | 1.0 |
|---|
| Creation Date | 2014-09-11 05:17:25 UTC |
|---|
| Update Date | 2016-11-09 01:09:13 UTC |
|---|
| Accession Number | CHEM003763 |
|---|
| Identification |
|---|
| Common Name | Aminoglutethimide |
|---|
| Class | Small Molecule |
|---|
| Description | An aromatase inhibitor that produces a state of 'medical' adrenalectomy by blocking the production of adrenal steroids. It also blocks the conversion of androgens to estrogens. Aminoglutethimide has been used in the treatment of advanced breast and prostate cancer. It was formerly used for its weak anticonvulsant properties. |
|---|
| Contaminant Sources | - HMDB Contaminants - Urine
- STOFF IDENT Compounds
- T3DB toxins
- ToxCast & Tox21 Chemicals
|
|---|
| Contaminant Type | - Amide
- Amine
- Antineoplastic Agent, Hormonal
- Aromatase Inhibitor
- Drug
- Metabolite
- Organic Compound
- Synthetic Compound
|
|---|
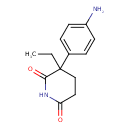
| Chemical Structure | |
|---|
| Synonyms | | Value | Source |
|---|
| 2-(p-Aminophenyl)-2-ethylglutarimide | ChEBI | | 3-Ethyl-3-(p-aminophenyl)-2,6-dioxopiperidine | ChEBI | | alpha-(p-Aminophenyl)-alpha-ethylglutarimide | ChEBI | | Aminoglutethimidum | ChEBI | | Aminoglutetimida | ChEBI | | DL-Aminoglutethimide | ChEBI | | p-Aminoglutethimide | ChEBI | | Cytadren | Kegg | | a-(p-Aminophenyl)-a-ethylglutarimide | Generator | | Α-(p-aminophenyl)-α-ethylglutarimide | Generator | | Ciba vision brand OF aminoglutethimide | HMDB | | Novartis brand OF aminoglutethimide | HMDB | | Orimeten | HMDB |
|
|---|
| Chemical Formula | C13H16N2O2 |
|---|
| Average Molecular Mass | 232.278 g/mol |
|---|
| Monoisotopic Mass | 232.121 g/mol |
|---|
| CAS Registry Number | 125-84-8 |
|---|
| IUPAC Name | 3-(4-aminophenyl)-3-ethylpiperidine-2,6-dione |
|---|
| Traditional Name | aminoglutethimide |
|---|
| SMILES | CCC1(CCC(=O)NC1=O)C1=CC=C(N)C=C1 |
|---|
| InChI Identifier | InChI=1S/C13H16N2O2/c1-2-13(8-7-11(16)15-12(13)17)9-3-5-10(14)6-4-9/h3-6H,2,7-8,14H2,1H3,(H,15,16,17) |
|---|
| InChI Key | ROBVIMPUHSLWNV-UHFFFAOYSA-N |
|---|
| Chemical Taxonomy |
|---|
| Description | belongs to the class of organic compounds known as aniline and substituted anilines. These are organic compounds containing an aminobenzene moiety. |
|---|
| Kingdom | Organic compounds |
|---|
| Super Class | Benzenoids |
|---|
| Class | Benzene and substituted derivatives |
|---|
| Sub Class | Aniline and substituted anilines |
|---|
| Direct Parent | Aniline and substituted anilines |
|---|
| Alternative Parents | |
|---|
| Substituents | - Aniline or substituted anilines
- Tetrahydropyridine
- Hydropyridine
- Amino acid or derivatives
- N-acylimine
- Lactim
- Organoheterocyclic compound
- Organic 1,3-dipolar compound
- Propargyl-type 1,3-dipolar organic compound
- Carboxylic acid derivative
- Azacycle
- Organopnictogen compound
- Amine
- Organic oxygen compound
- Primary amine
- Organooxygen compound
- Organonitrogen compound
- Organic nitrogen compound
- Carbonyl group
- Hydrocarbon derivative
- Organic oxide
- Aromatic heteromonocyclic compound
|
|---|
| Molecular Framework | Aromatic heteromonocyclic compounds |
|---|
| External Descriptors | |
|---|
| Biological Properties |
|---|
| Status | Detected and Not Quantified |
|---|
| Origin | Exogenous |
|---|
| Cellular Locations | - Cytoplasm
- Extracellular
- Membrane
|
|---|
| Biofluid Locations | Not Available |
|---|
| Tissue Locations | Not Available |
|---|
| Pathways | Not Available |
|---|
| Applications | Not Available |
|---|
| Biological Roles | Not Available |
|---|
| Chemical Roles | Not Available |
|---|
| Physical Properties |
|---|
| State | Solid |
|---|
| Appearance | White powder. |
|---|
| Experimental Properties | | Property | Value |
|---|
| Melting Point | 223-225°C | | Boiling Point | Not Available | | Solubility | Practically insoluble in water |
|
|---|
| Predicted Properties | |
|---|
| Spectra |
|---|
| Spectra | | Spectrum Type | Description | Splash Key | View |
|---|
| GC-MS | GC-MS Spectrum - EI-B (Non-derivatized) | splash10-0ue9-5960000000-f9b33cb2d18e6b01ae76 | Spectrum | | GC-MS | GC-MS Spectrum - EI-B (Non-derivatized) | splash10-0ue9-5960000000-f9b33cb2d18e6b01ae76 | Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | splash10-01q9-0930000000-1559858da10d4930e919 | Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | Not Available | Spectrum | | LC-MS/MS | LC-MS/MS Spectrum - LC-ESI-qTof , Positive | splash10-0540-2960000000-949d8dddc3981b8c3e63 | Spectrum | | LC-MS/MS | LC-MS/MS Spectrum - LC-ESI-qTof , Positive | splash10-0uxr-0905000000-155a653652a8dc132b5c | Spectrum | | LC-MS/MS | LC-MS/MS Spectrum - LC-ESI-qTof , Positive | splash10-001j-3910000000-d92bbd37c74ab8b7828d | Spectrum | | LC-MS/MS | LC-MS/MS Spectrum - , positive | splash10-0540-1970000000-fc41c032a79d79173387 | Spectrum | | LC-MS/MS | LC-MS/MS Spectrum - , positive | splash10-0540-2960000000-949d8dddc3981b8c3e63 | Spectrum | | LC-MS/MS | LC-MS/MS Spectrum - , positive | splash10-001j-3910000000-d92bbd37c74ab8b7828d | Spectrum | | LC-MS/MS | LC-MS/MS Spectrum - 30V, Positive | splash10-00di-0910000000-2af28058ce65d2c2b40e | Spectrum | | LC-MS/MS | LC-MS/MS Spectrum - 15V, Positive | splash10-00di-0920000000-a50aa2833e911342e507 | Spectrum | | LC-MS/MS | LC-MS/MS Spectrum - 45V, Positive | splash10-00di-0900000000-d172683054220fdf2313 | Spectrum | | LC-MS/MS | LC-MS/MS Spectrum - 60V, Positive | splash10-05fr-0900000000-3564a0e475748f410e23 | Spectrum | | LC-MS/MS | LC-MS/MS Spectrum - 30V, Negative | splash10-014i-0290000000-99e0c515559b4638490a | Spectrum | | LC-MS/MS | LC-MS/MS Spectrum - 15V, Negative | splash10-0159-0090000000-22908f241db3fc37cdbc | Spectrum | | LC-MS/MS | LC-MS/MS Spectrum - 75V, Positive | splash10-0a59-0900000000-6a7e540e50c50942e829 | Spectrum | | LC-MS/MS | LC-MS/MS Spectrum - 90V, Positive | splash10-001i-0900000000-3c4030cf08f3c992fdac | Spectrum | | LC-MS/MS | LC-MS/MS Spectrum - 90V, Negative | splash10-0006-0900000000-8be298c9d42d7769f129 | Spectrum | | LC-MS/MS | LC-MS/MS Spectrum - 75V, Negative | splash10-0006-0900000000-a290024caadd7d64fe8f | Spectrum | | LC-MS/MS | LC-MS/MS Spectrum - 45V, Negative | splash10-00kf-0940000000-ce0f2ff21762621367e5 | Spectrum | | LC-MS/MS | LC-MS/MS Spectrum - 60V, Negative | splash10-0006-0900000000-24977ebbbf8d80633142 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-00lr-0190000000-bd92b28e06f9a2c94ab1 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-00lr-0590000000-2ecccc66bf459b9dff9b | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-00l2-2900000000-486145b0245b40ea818c | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-001i-0090000000-b5c20a34adc0796f60c2 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-01q9-1190000000-e50243f982300e3ad740 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-0006-9410000000-a3860f54f1f49a280fb8 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-001i-0090000000-62df4688c2e6b97f190f | Spectrum | | MS | Mass Spectrum (Electron Ionization) | splash10-0f89-3950000000-013571ce504af9a2d603 | Spectrum |
|
|---|
| Toxicity Profile |
|---|
| Route of Exposure | Rapidly and completely absorbed from gastrointestinal tract. The bioavailability of tablets is equivalent to equal doses given as a solution. |
|---|
| Mechanism of Toxicity | Aminoglutethimide reduces the production of D5-pregnenolone and blocks several other steps in steroid synthesis, including the C-11, C-18, and C-21 hydroxylations and the hydroxylations required for the aromatization of androgens to estrogens, mediated through the binding of aminoglutethimide to cytochrome P-450 complexes. Specifically, the drug binds to and inhibits aromatase which is essential for the generation of estrogens from androstenedione and testosterone. A decrease in adrenal secretion of cortisol is followed by an increased secretion of pituitary adrenocorticotropic hormone (ACTH), which will overcome the blockade of adrenocortical steroid synthesis by aminoglutethimide. The compensatory increase in ACTH secretion can be suppressed by the simultaneous administration of hydrocortisone. Since aminoglutethimide increases the rate of metabolism of dexamethasone but not that of hydrocortisone, the latter is preferred as the adrenal glucocorticoid replacement. Although aminoglutethimide inhibits the synthesis of thyroxine by the thyroid gland, the compensatory increase in thyroid-stimulating hormone (TSH) is frequently of sufficient magnitude to overcome the inhibition of thyroid synthesis due to aminoglutethimide. In spite of an increase in TSH, aminoglutethimide has not been associated with increased prolactin secretion. |
|---|
| Metabolism | Hepatic. 34-54% of the administered dose is excreted in the urine as unchanged drug during the first 48 hours, and an additional fraction as an N-acetyl derivative.
Route of Elimination: After ingestion of a single oral dose, 34%-54% is excreted in the urine as unchanged drug during the first 48 hours, and an additional fraction as the N-acetyl derivative.
Half Life: 12.5 ± 1.6 hours |
|---|
| Toxicity Values | Oral LD50s (mg/kg): rats, 1800; dogs, >100. Intravenous LD50s (mg/kg): rats, 156; dogs, >100. |
|---|
| Lethal Dose | Not Available |
|---|
| Carcinogenicity (IARC Classification) | No indication of carcinogenicity to humans (not listed by IARC). |
|---|
| Uses/Sources | For the suppression of adrenal function in selected patients with Cushing's syndrome, malignant neoplasm of the female breast, and carcinoma in situ of the breast. |
|---|
| Minimum Risk Level | Not Available |
|---|
| Health Effects | Not Available |
|---|
| Symptoms | Symptoms of overdose include respiratory depression, hypoventilation, hypotension, hypovolemic shock due to dehydration, somnolence, lethargy, coma, ataxia, dizziness, fatigue, nausea, and vomiting. |
|---|
| Treatment | Not Available |
|---|
| Concentrations |
|---|
| Not Available |
|---|
| External Links |
|---|
| DrugBank ID | DB00357 |
|---|
| HMDB ID | HMDB0014501 |
|---|
| FooDB ID | Not Available |
|---|
| Phenol Explorer ID | Not Available |
|---|
| KNApSAcK ID | Not Available |
|---|
| BiGG ID | Not Available |
|---|
| BioCyc ID | Not Available |
|---|
| METLIN ID | Not Available |
|---|
| PDB ID | Not Available |
|---|
| Wikipedia Link | Aminoglutethimide |
|---|
| Chemspider ID | 2060 |
|---|
| ChEBI ID | 2654 |
|---|
| PubChem Compound ID | 2145 |
|---|
| Kegg Compound ID | C07617 |
|---|
| YMDB ID | Not Available |
|---|
| ECMDB ID | Not Available |
|---|
| References |
|---|
| Synthesis Reference | Hoffmann, K.and Urech, E.; U.S. Patent 2,848,455; August 19,1958; assigned to Ciba Pharmaceutical Products, Inc. |
|---|
| MSDS | Not Available |
|---|
| General References | |
|---|