| Record Information |
|---|
| Version | 1.0 |
|---|
| Creation Date | 2014-09-11 05:16:50 UTC |
|---|
| Update Date | 2016-11-09 01:09:12 UTC |
|---|
| Accession Number | CHEM003748 |
|---|
| Identification |
|---|
| Common Name | Mestranol |
|---|
| Class | Small Molecule |
|---|
| Description | The 3-methyl ether of ethinyl estradiol. It must be demethylated to be biologically active. It is used as the estrogen component of many combination ORAL contraceptives. |
|---|
| Contaminant Sources | - HMDB Contaminants - Urine
- STOFF IDENT Compounds
- T3DB toxins
- ToxCast & Tox21 Chemicals
|
|---|
| Contaminant Type | - Drug
- Ether
- Metabolite
- Organic Compound
- Synthetic Compound
|
|---|
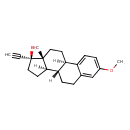
| Chemical Structure | |
|---|
| Synonyms | Not Available |
|---|
| Chemical Formula | C21H26O2 |
|---|
| Average Molecular Mass | 310.437 g/mol |
|---|
| Monoisotopic Mass | 310.193 g/mol |
|---|
| CAS Registry Number | 72-33-3 |
|---|
| IUPAC Name | (1R,10S,11R,14S,15R)-14-ethynyl-5-methoxy-15-methyltetracyclo[8.7.0.0²,⁷.0¹¹,¹⁵]heptadeca-2,4,6-trien-14-ol |
|---|
| Traditional Name | (1R,10S,11R,14S,15R)-14-ethynyl-5-methoxy-15-methyltetracyclo[8.7.0.0²,⁷.0¹¹,¹⁵]heptadeca-2,4,6-trien-14-ol |
|---|
| SMILES | [H][C@]12CC[C@](O)(C#C)[C@]1(C)CC[C@@]1([H])C3=CC=C(OC)C=C3CC[C@]21[H] |
|---|
| InChI Identifier | InChI=1S/C21H26O2/c1-4-21(22)12-10-19-18-7-5-14-13-15(23-3)6-8-16(14)17(18)9-11-20(19,21)2/h1,6,8,13,17-19,22H,5,7,9-12H2,2-3H3/t17-,18-,19+,20+,21+/m0/s1 |
|---|
| InChI Key | IMSSROKUHAOUJS-WRTQPQOASA-N |
|---|
| Chemical Taxonomy |
|---|
| Description | belongs to the class of organic compounds known as estrane steroids. These are steroids with a structure based on the estrane skeleton. |
|---|
| Kingdom | Organic compounds |
|---|
| Super Class | Lipids and lipid-like molecules |
|---|
| Class | Steroids and steroid derivatives |
|---|
| Sub Class | Estrane steroids |
|---|
| Direct Parent | Estrane steroids |
|---|
| Alternative Parents | |
|---|
| Substituents | - 17-hydroxysteroid
- Hydroxysteroid
- Estrane-skeleton
- Phenanthrene
- Tetralin
- Anisole
- Alkyl aryl ether
- Benzenoid
- Ynone
- Tertiary alcohol
- Cyclic alcohol
- Acetylide
- Ether
- Organic oxygen compound
- Hydrocarbon derivative
- Organooxygen compound
- Alcohol
- Aromatic homopolycyclic compound
|
|---|
| Molecular Framework | Aromatic homopolycyclic compounds |
|---|
| External Descriptors | Not Available |
|---|
| Biological Properties |
|---|
| Status | Detected and Not Quantified |
|---|
| Origin | Exogenous |
|---|
| Cellular Locations | - Cytoplasm
- Extracellular
- Membrane
|
|---|
| Biofluid Locations | Not Available |
|---|
| Tissue Locations | Not Available |
|---|
| Pathways | Not Available |
|---|
| Applications | Not Available |
|---|
| Biological Roles | Not Available |
|---|
| Chemical Roles | Not Available |
|---|
| Physical Properties |
|---|
| State | Solid |
|---|
| Appearance | White powder. |
|---|
| Experimental Properties | | Property | Value |
|---|
| Melting Point | 150.5°C | | Boiling Point | Not Available | | Solubility | 3.77e-03 g/L |
|
|---|
| Predicted Properties | |
|---|
| Spectra |
|---|
| Spectra | | Spectrum Type | Description | Splash Key | View |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-03di-0149000000-c1a392ac6a255cba0e08 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-03dr-0592000000-0cc5cea78731703ef0b3 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-0k9f-3980000000-fe6e57c0238f233c2ba6 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-0a4i-0009000000-85dc48ac6fb3820afe06 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-0a4i-0069000000-cd914e79f9bb96f842c3 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-0f76-0090000000-84d635c97eb379340fca | Spectrum |
|
|---|
| Toxicity Profile |
|---|
| Route of Exposure | Not Available |
|---|
| Mechanism of Toxicity | Mestranol is the 3-methyl ether of ethinylestradiol. Ethinylestradiol, is a synthetic derivative of estradiol. Ethinylestradiol is orally bio-active and the estrogen used in almost all modern formulations of combined oral contraceptive pills. It binds to (and activates) the estrogen receptor. Mestranol is a biologically inactive prodrug of ethinylestradiol to which it is demethylated in the liver with a conversion efficiency of 70%.
Estrogens diffuse into their target cells and interact with a protein receptor. Target cells include the female reproductive tract, the mammary gland, the hypothalamus, and the pituitary. Estrogens increase the hepatic synthesis of sex hormone binding globulin (SHBG), thyroid-binding globulin (TBG), and other serum proteins and suppress follicle-stimulating hormone (FSH) from the anterior pituitary. The combination of an estrogen with a progestin suppresses the hypothalamic-pituitary system, decreasing the secretion of gonadotropin-releasing hormone (GnRH). |
|---|
| Metabolism | Not Available |
|---|
| Toxicity Values | Not Available |
|---|
| Lethal Dose | Not Available |
|---|
| Carcinogenicity (IARC Classification) | No indication of carcinogenicity to humans (not listed by IARC). |
|---|
| Uses/Sources | Mestranol was used as one of the first oral contraceptives. |
|---|
| Minimum Risk Level | Not Available |
|---|
| Health Effects | Not Available |
|---|
| Symptoms | Not Available |
|---|
| Treatment | Not Available |
|---|
| Concentrations |
|---|
| Not Available |
|---|
| External Links |
|---|
| DrugBank ID | Not Available |
|---|
| HMDB ID | Not Available |
|---|
| FooDB ID | Not Available |
|---|
| Phenol Explorer ID | Not Available |
|---|
| KNApSAcK ID | Not Available |
|---|
| BiGG ID | Not Available |
|---|
| BioCyc ID | Not Available |
|---|
| METLIN ID | Not Available |
|---|
| PDB ID | Not Available |
|---|
| Wikipedia Link | Not Available |
|---|
| Chemspider ID | Not Available |
|---|
| ChEBI ID | Not Available |
|---|
| PubChem Compound ID | 12598371 |
|---|
| Kegg Compound ID | Not Available |
|---|
| YMDB ID | Not Available |
|---|
| ECMDB ID | Not Available |
|---|
| References |
|---|
| Synthesis Reference | Not Available |
|---|
| MSDS | Not Available |
|---|
| General References | Not Available |
|---|