| Record Information |
|---|
| Version | 1.0 |
|---|
| Creation Date | 2014-09-11 05:15:56 UTC |
|---|
| Update Date | 2016-11-09 01:09:12 UTC |
|---|
| Accession Number | CHEM003729 |
|---|
| Identification |
|---|
| Common Name | Stavudine |
|---|
| Class | Small Molecule |
|---|
| Description | A dideoxynucleoside analog that inhibits reverse transcriptase and has in vitro activity against HIV. |
|---|
| Contaminant Sources | - HMDB Contaminants - Urine
- STOFF IDENT Compounds
- T3DB toxins
- ToxCast & Tox21 Chemicals
|
|---|
| Contaminant Type | - Amide
- Anti-HIV Agent
- Antimetabolite
- Drug
- Ether
- Metabolite
- Organic Compound
- Reverse Transcriptase Inhibitor
- Synthetic Compound
|
|---|
| Chemical Structure | |
|---|
| Synonyms | | Value | Source |
|---|
| 1-(2,3-Dideoxy-beta-D-glycero-pent-2-enofuranosyl)thymine | ChEBI | | 2',3'-Didehydro-3'-deoxythimidine | ChEBI | | 3'-Deoxy-2'-thymidinene | ChEBI | | d4T | ChEBI | | Estavudina | ChEBI | | Sanilvudine | ChEBI | | Stavudinum | ChEBI | | STV | ChEBI | | Zerit | Kegg | | ABBR D4T | Kegg | | 1-(2,3-Dideoxy-b-D-glycero-pent-2-enofuranosyl)thymine | Generator | | 1-(2,3-Dideoxy-β-D-glycero-pent-2-enofuranosyl)thymine | Generator | | 2',3'-Didehydro-2',3'-dideoxythmidine | HMDB | | 2',3' Didehydro 3' deoxythymidine | HMDB | | 2',3'-Didehydro-3'-deoxythymidine | HMDB | | Bristol-myers squibb brand OF stavudine | HMDB | | Bristol-myers brand OF stavudine | HMDB | | Stavudine, monosodium salt | HMDB |
|
|---|
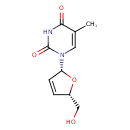
| Chemical Formula | C10H12N2O4 |
|---|
| Average Molecular Mass | 224.213 g/mol |
|---|
| Monoisotopic Mass | 224.080 g/mol |
|---|
| CAS Registry Number | 3056-17-5 |
|---|
| IUPAC Name | 1-[(2R,5S)-5-(hydroxymethyl)-2,5-dihydrofuran-2-yl]-5-methyl-1,2,3,4-tetrahydropyrimidine-2,4-dione |
|---|
| Traditional Name | stavudine |
|---|
| SMILES | CC1=CN([C@@H]2O[C@H](CO)C=C2)C(=O)NC1=O |
|---|
| InChI Identifier | InChI=1S/C10H12N2O4/c1-6-4-12(10(15)11-9(6)14)8-3-2-7(5-13)16-8/h2-4,7-8,13H,5H2,1H3,(H,11,14,15)/t7-,8+/m0/s1 |
|---|
| InChI Key | XNKLLVCARDGLGL-JGVFFNPUSA-N |
|---|
| Chemical Taxonomy |
|---|
| Description | belongs to the class of organic compounds known as nucleoside and nucleotide analogues. These are analogues of nucleosides and nucleotides. These include phosphonated nucleosides, C-glycosylated nucleoside bases, analogues where the sugar unit is a pyranose, and carbocyclic nucleosides, among others. |
|---|
| Kingdom | Organic compounds |
|---|
| Super Class | Nucleosides, nucleotides, and analogues |
|---|
| Class | Nucleoside and nucleotide analogues |
|---|
| Sub Class | Not Available |
|---|
| Direct Parent | Nucleoside and nucleotide analogues |
|---|
| Alternative Parents | |
|---|
| Substituents | - Pyrimidone
- Hydropyrimidine
- Pyrimidine
- Dihydrofuran
- Vinylogous amide
- Heteroaromatic compound
- Lactam
- Urea
- Organoheterocyclic compound
- Azacycle
- Oxacycle
- Hydrocarbon derivative
- Organic oxide
- Organopnictogen compound
- Primary alcohol
- Organooxygen compound
- Organonitrogen compound
- Alcohol
- Organic oxygen compound
- Organic nitrogen compound
- Aromatic heteromonocyclic compound
|
|---|
| Molecular Framework | Aromatic heteromonocyclic compounds |
|---|
| External Descriptors | |
|---|
| Biological Properties |
|---|
| Status | Detected and Not Quantified |
|---|
| Origin | Exogenous |
|---|
| Cellular Locations | |
|---|
| Biofluid Locations | Not Available |
|---|
| Tissue Locations | Not Available |
|---|
| Pathways | Not Available |
|---|
| Applications | Not Available |
|---|
| Biological Roles | Not Available |
|---|
| Chemical Roles | Not Available |
|---|
| Physical Properties |
|---|
| State | Solid |
|---|
| Appearance | White powder. |
|---|
| Experimental Properties | | Property | Value |
|---|
| Melting Point | 159-160°C | | Boiling Point | Not Available | | Solubility | 5-10 g/100 mL at 21°C |
|
|---|
| Predicted Properties | |
|---|
| Spectra |
|---|
| Spectra | | Spectrum Type | Description | Splash Key | View |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | splash10-004i-9710000000-ec1b8accee7609d895af | Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (1 TMS) - 70eV, Positive | splash10-0fi9-9710000000-a08eaef1e27e4362b56e | Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | Not Available | Spectrum | | LC-MS/MS | LC-MS/MS Spectrum - 15V, Negative | splash10-00di-0970000000-1b5ba27caa3ca46a5b79 | Spectrum | | LC-MS/MS | LC-MS/MS Spectrum - 30V, Negative | splash10-0fk9-0910000000-0c32425ddc6552e762c7 | Spectrum | | LC-MS/MS | LC-MS/MS Spectrum - 90V, Positive | splash10-053i-1900000000-8995ae3343a9f5bbbd7e | Spectrum | | LC-MS/MS | LC-MS/MS Spectrum - 75V, Positive | splash10-052r-1900000000-b232bf6bd07c73125776 | Spectrum | | LC-MS/MS | LC-MS/MS Spectrum - 35V, Negative | splash10-0006-9000000000-b6b8d8a7acd0cee4a6be | Spectrum | | LC-MS/MS | LC-MS/MS Spectrum - 35V, Positive | splash10-004i-0900000000-bfecc35d096bfac41070 | Spectrum | | LC-MS/MS | LC-MS/MS Spectrum - 45V, Positive | splash10-014r-0900000000-7eaff645bdf6c4509baf | Spectrum | | LC-MS/MS | LC-MS/MS Spectrum - 30V, Positive | splash10-014i-0910000000-14958ea9ed30262a4313 | Spectrum | | LC-MS/MS | LC-MS/MS Spectrum - 75V, Negative | splash10-0a4i-0900000000-f6122db2223358609e31 | Spectrum | | LC-MS/MS | LC-MS/MS Spectrum - 90V, Negative | splash10-0a4i-0900000000-c3ebc31e7ad70d77da44 | Spectrum | | LC-MS/MS | LC-MS/MS Spectrum - 60V, Negative | splash10-0a4i-0900000000-d5d5a96f8199343194ce | Spectrum | | LC-MS/MS | LC-MS/MS Spectrum - 45V, Negative | splash10-0a4i-0900000000-2e40076ad350727ab3ef | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-004i-0900000000-a15778802feedb7e8702 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-004i-5900000000-1e77dd699ba1244d96dd | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-0a7j-9600000000-42ce2a756eceb750ac46 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-00c0-2940000000-3e2b43ac1b4b3b11d540 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-01di-3920000000-0a634444532bc0df9549 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-0006-9200000000-5a3e9da67f3d054c39c8 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-004i-0910000000-3590697dc556ba789bc3 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-004i-4930000000-0d37f8daf0a0e84719bf | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-0a59-9400000000-0f2472d56e156d73d0de | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-00di-0090000000-2b6aa9078cee3df72a6f | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-00bc-7960000000-a5ae84e7332328ddeee2 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-0006-9100000000-22b5a0eeff96c030db6a | Spectrum |
|
|---|
| Toxicity Profile |
|---|
| Route of Exposure | Following oral administration, stavudine is rapidly absorbed (bioavailability is 68-104%). |
|---|
| Mechanism of Toxicity | Stavudine inhibits the activity of HIV-1 reverse transcriptase (RT) both by competing with the natural substrate dGTP and by its incorporation into viral DNA. |
|---|
| Metabolism | Phosphorylated intracellularly to stavudine triphosphate, the active substrate for HIV-reverse transcriptase.
Half Life: 0.8-1.5 hours (in adults) |
|---|
| Toxicity Values | Not Available |
|---|
| Lethal Dose | Not Available |
|---|
| Carcinogenicity (IARC Classification) | No indication of carcinogenicity to humans (not listed by IARC). |
|---|
| Uses/Sources | For the treatment of human immunovirus (HIV) infections. |
|---|
| Minimum Risk Level | Not Available |
|---|
| Health Effects | Not Available |
|---|
| Symptoms | Side effects include peripheral neuropathy tingling, burning, numbness, or pain in the hands or feet), fatal lactic acidosis has been reported in patients treated with stavudine (ZERIT) in combination with other antiretroviral agents, severe liver enlargement, inflammation (pain and swelling) of the liver, and liver failure. |
|---|
| Treatment | Not Available |
|---|
| Concentrations |
|---|
| Not Available |
|---|
| External Links |
|---|
| DrugBank ID | DB00649 |
|---|
| HMDB ID | HMDB0014787 |
|---|
| FooDB ID | Not Available |
|---|
| Phenol Explorer ID | Not Available |
|---|
| KNApSAcK ID | Not Available |
|---|
| BiGG ID | Not Available |
|---|
| BioCyc ID | Not Available |
|---|
| METLIN ID | Not Available |
|---|
| PDB ID | Not Available |
|---|
| Wikipedia Link | Stavudine |
|---|
| Chemspider ID | 17270 |
|---|
| ChEBI ID | 63581 |
|---|
| PubChem Compound ID | 18283 |
|---|
| Kegg Compound ID | C07312 |
|---|
| YMDB ID | Not Available |
|---|
| ECMDB ID | Not Available |
|---|
| References |
|---|
| Synthesis Reference | Purna Chandra Ray, Jagan Mohana Chary Tummanapalli, Seeta Ramanjaneyulu Gorantla, “Process for the Large Scale Production of Stavudine.” U.S. Patent US20080312428, issued December 18, 2008. |
|---|
| MSDS | Link |
|---|
| General References | Not Available |
|---|