| Record Information |
|---|
| Version | 1.0 |
|---|
| Creation Date | 2014-09-11 05:15:03 UTC |
|---|
| Update Date | 2016-11-09 01:09:12 UTC |
|---|
| Accession Number | CHEM003713 |
|---|
| Identification |
|---|
| Common Name | Triamcinolone |
|---|
| Class | Small Molecule |
|---|
| Description | A glucocorticoid given, as the free alcohol or in esterified form, orally, intramuscularly, by local injection, by inhalation, or applied topically in the management of various disorders in which corticosteroids are indicated. |
|---|
| Contaminant Sources | - HMDB Contaminants - Urine
- STOFF IDENT Compounds
- T3DB toxins
- ToxCast & Tox21 Chemicals
|
|---|
| Contaminant Type | - Anti-Inflammatory Agent
- Drug
- Ester
- Glucocorticoid
- Metabolite
- Organic Compound
- Organofluoride
- Synthetic Compound
|
|---|
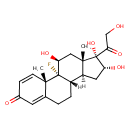
| Chemical Structure | |
|---|
| Synonyms | | Value | Source |
|---|
| 11beta,16alpha,17alpha,21-Tetrahydroxy-9alpha-fluoro-1,4-pregnadiene-3,20-dione | ChEBI | | 9-Fluoro-11beta,16alpha,17,21-tetrahydroxypregna-1,4-diene-3,20-dione | ChEBI | | 9alpha-Fluoro-11beta,16alpha,17,21-tetrahydroxypregna-1,4-diene-3,20-dione | ChEBI | | 9alpha-Fluoro-11beta,16alpha,17alpha,21-tetrahydroxypregna-1,4-diene-3,20-dione | ChEBI | | 9alpha-Fluoro-16alpha-hydroxyprednisolone | ChEBI | | Fluoxyprednisolone | ChEBI | | Triamcinolona | ChEBI | | Triamcinolonum | ChEBI | | Aristocort | Kegg | | Kenacort | Kegg | | 11b,16a,17a,21-Tetrahydroxy-9a-fluoro-1,4-pregnadiene-3,20-dione | Generator | | 11Β,16α,17α,21-tetrahydroxy-9α-fluoro-1,4-pregnadiene-3,20-dione | Generator | | 9-Fluoro-11b,16a,17,21-tetrahydroxypregna-1,4-diene-3,20-dione | Generator | | 9-Fluoro-11β,16α,17,21-tetrahydroxypregna-1,4-diene-3,20-dione | Generator | | 9a-Fluoro-11b,16a,17,21-tetrahydroxypregna-1,4-diene-3,20-dione | Generator | | 9Α-fluoro-11β,16α,17,21-tetrahydroxypregna-1,4-diene-3,20-dione | Generator | | 9a-Fluoro-11b,16a,17a,21-tetrahydroxypregna-1,4-diene-3,20-dione | Generator | | 9Α-fluoro-11β,16α,17α,21-tetrahydroxypregna-1,4-diene-3,20-dione | Generator | | 9a-Fluoro-16a-hydroxyprednisolone | Generator | | 9Α-fluoro-16α-hydroxyprednisolone | Generator | | Fluoxiprednisolone | HMDB | | Triamcinalone | HMDB | | Triamcinolone acetonide | HMDB | | Triamcinolone diacetate | HMDB | | Triamcinolone hexacetonide | HMDB | | Volon | HMDB |
|
|---|
| Chemical Formula | C21H27FO6 |
|---|
| Average Molecular Mass | 394.434 g/mol |
|---|
| Monoisotopic Mass | 394.179 g/mol |
|---|
| CAS Registry Number | 124-94-7 |
|---|
| IUPAC Name | (1R,2S,10S,11S,13R,14S,15S,17S)-1-fluoro-13,14,17-trihydroxy-14-(2-hydroxyacetyl)-2,15-dimethyltetracyclo[8.7.0.0^{2,7}.0^{11,15}]heptadeca-3,6-dien-5-one |
|---|
| Traditional Name | (1R,2S,10S,11S,13R,14S,15S,17S)-1-fluoro-13,14,17-trihydroxy-14-(2-hydroxyacetyl)-2,15-dimethyltetracyclo[8.7.0.0^{2,7}.0^{11,15}]heptadeca-3,6-dien-5-one |
|---|
| SMILES | [H][C@@]12C[C@@H](O)[C@](O)(C(=O)CO)[C@@]1(C)C[C@H](O)[C@@]1(F)[C@@]2([H])CCC2=CC(=O)C=C[C@]12C |
|---|
| InChI Identifier | InChI=1S/C21H27FO6/c1-18-6-5-12(24)7-11(18)3-4-13-14-8-15(25)21(28,17(27)10-23)19(14,2)9-16(26)20(13,18)22/h5-7,13-16,23,25-26,28H,3-4,8-10H2,1-2H3/t13-,14-,15+,16-,18-,19-,20-,21-/m0/s1 |
|---|
| InChI Key | GFNANZIMVAIWHM-OBYCQNJPSA-N |
|---|
| Chemical Taxonomy |
|---|
| Description | belongs to the class of organic compounds known as 21-hydroxysteroids. These are steroids carrying a hydroxyl group at the 21-position of the steroid backbone. |
|---|
| Kingdom | Organic compounds |
|---|
| Super Class | Lipids and lipid-like molecules |
|---|
| Class | Steroids and steroid derivatives |
|---|
| Sub Class | Hydroxysteroids |
|---|
| Direct Parent | 21-hydroxysteroids |
|---|
| Alternative Parents | |
|---|
| Substituents | - Progestogin-skeleton
- 21-hydroxysteroid
- Pregnane-skeleton
- 20-oxosteroid
- 3-oxo-delta-1,4-steroid
- 3-oxosteroid
- 9-halo-steroid
- 17-hydroxysteroid
- 16-hydroxysteroid
- 11-hydroxysteroid
- 16-alpha-hydroxysteroid
- 11-beta-hydroxysteroid
- Halo-steroid
- Oxosteroid
- Delta-1,4-steroid
- Alpha-hydroxy ketone
- Cyclic alcohol
- Tertiary alcohol
- Fluorohydrin
- Cyclic ketone
- Halohydrin
- Secondary alcohol
- Ketone
- Polyol
- Organic oxide
- Hydrocarbon derivative
- Organic oxygen compound
- Organooxygen compound
- Carbonyl group
- Primary alcohol
- Alkyl halide
- Organofluoride
- Alcohol
- Alkyl fluoride
- Organohalogen compound
- Aliphatic homopolycyclic compound
|
|---|
| Molecular Framework | Aliphatic homopolycyclic compounds |
|---|
| External Descriptors | |
|---|
| Biological Properties |
|---|
| Status | Detected and Not Quantified |
|---|
| Origin | Exogenous |
|---|
| Cellular Locations | - Cytoplasm
- Extracellular
- Membrane
|
|---|
| Biofluid Locations | Not Available |
|---|
| Tissue Locations | Not Available |
|---|
| Pathways | Not Available |
|---|
| Applications | Not Available |
|---|
| Biological Roles | Not Available |
|---|
| Chemical Roles | Not Available |
|---|
| Physical Properties |
|---|
| State | Solid |
|---|
| Appearance | White powder. |
|---|
| Experimental Properties | | Property | Value |
|---|
| Melting Point | 270°C | | Boiling Point | Not Available | | Solubility | 80 mg/L (at 25°C) |
|
|---|
| Predicted Properties | |
|---|
| Spectra |
|---|
| Spectra | | Spectrum Type | Description | Splash Key | View |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | splash10-05q9-4958000000-8d5e298bc84911e269f1 | Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (4 TMS) - 70eV, Positive | splash10-014i-1304009000-a6db8dfa34bd6ccf8bd6 | Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | Not Available | Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | Not Available | Spectrum | | LC-MS/MS | LC-MS/MS Spectrum - LC-ESI-qTof , Positive | splash10-006t-3970000000-70c8dcf068945a18ecdd | Spectrum | | LC-MS/MS | LC-MS/MS Spectrum - , positive | splash10-006t-3970000000-70c8dcf068945a18ecdd | Spectrum | | LC-MS/MS | LC-MS/MS Spectrum - 30V, Negative | splash10-002b-0009000000-139556289d74866f2be3 | Spectrum | | LC-MS/MS | LC-MS/MS Spectrum - 20V, Positive | splash10-0a70-0296000000-07efad54f91e2f4f8da9 | Spectrum | | LC-MS/MS | LC-MS/MS Spectrum - 10V, Positive | splash10-004j-0019000000-2a242af1cc09f059e525 | Spectrum | | LC-MS/MS | LC-MS/MS Spectrum - 20V, Negative | splash10-0002-0009000000-8c3845c2bd0bb91f6658 | Spectrum | | LC-MS/MS | LC-MS/MS Spectrum - 90V, Positive | splash10-00mo-2910000000-8b5e71de002c589e636a | Spectrum | | LC-MS/MS | LC-MS/MS Spectrum - 30V, Positive | splash10-020a-0490000000-0f3f7ce5872fa7f7533a | Spectrum | | LC-MS/MS | LC-MS/MS Spectrum - 40V, Positive | splash10-0592-0790000000-f2eacbf26900597c87ff | Spectrum | | LC-MS/MS | LC-MS/MS Spectrum - 10V, Negative | splash10-0002-0009000000-471ea538b5d986e0c4df | Spectrum | | LC-MS/MS | LC-MS/MS Spectrum - 45V, Positive | splash10-00dj-1960000000-5d9e6e746dc765305042 | Spectrum | | LC-MS/MS | LC-MS/MS Spectrum - 15V, Negative | splash10-0002-0009000000-ce41bd6a5080c4c10c1c | Spectrum | | LC-MS/MS | LC-MS/MS Spectrum - 50V, Positive | splash10-0592-0960000000-cd971a61830ca6619b63 | Spectrum | | LC-MS/MS | LC-MS/MS Spectrum - 45V, Positive | splash10-00dj-1960000000-b64d7111d5289725129b | Spectrum | | LC-MS/MS | LC-MS/MS Spectrum - 30V, Positive | splash10-01rj-0491000000-c7e04bfdda68e0cbc0a7 | Spectrum | | LC-MS/MS | LC-MS/MS Spectrum - 40V, Negative | splash10-004i-0009000000-a7af8c93633183e5865e | Spectrum | | LC-MS/MS | LC-MS/MS Spectrum - 30V, Negative | splash10-002b-0009000000-9f249447972141b9bceb | Spectrum | | LC-MS/MS | LC-MS/MS Spectrum - 50V, Positive | splash10-0592-0960000000-a77f2165c6e21f53b6c3 | Spectrum | | LC-MS/MS | LC-MS/MS Spectrum - 15V, Positive | splash10-0a4r-0279000000-2f221b536c1c36be4e8c | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-004j-0009000000-7c85eb6f9e8e9630084d | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-0a4i-6039000000-53d51d2a92776995454c | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-0ap0-2194000000-072453c8d1ed2f28f6f8 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-000f-0009000000-35cd67a7424fc78754b7 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-0a4i-4009000000-2aa7a537a1470cde7169 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-0aou-6049000000-68d446beab8eb3cdcbcc | Spectrum | | MS | Mass Spectrum (Electron Ionization) | splash10-00di-2931000000-ee37afbf8c285181a340 | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum |
|
|---|
| Toxicity Profile |
|---|
| Route of Exposure | Rapid absorption following oral administration |
|---|
| Mechanism of Toxicity | The antiinflammatory actions of corticosteroids are thought to involve lipocortins, phospholipase A2 inhibitory proteins which, through inhibition of arachidonic acid, control the biosynthesis of prostaglandins and leukotrienes. Firstly, however, these glucocorticoids bind to the glucocorticoid receptors which translocate into the nucleus and bind DNA (GRE) and change genetic expression both positively and negatively. The immune system is suppressed by corticosteroids due to a decrease in the function of the lymphatic system, a reduction in immunoglobulin and complement concentrations, the precipitation of lymphocytopenia, and interference with antigen-antibody binding. |
|---|
| Metabolism | Hepatic.
Half Life: 88 minutes |
|---|
| Toxicity Values | LD50=>500mg/kg (in rats) |
|---|
| Lethal Dose | Not Available |
|---|
| Carcinogenicity (IARC Classification) | No indication of carcinogenicity to humans (not listed by IARC). |
|---|
| Uses/Sources | For the treatment of perennial and seasonal allergic rhinitis. |
|---|
| Minimum Risk Level | Not Available |
|---|
| Health Effects | Not Available |
|---|
| Symptoms | Not Available |
|---|
| Treatment | Not Available |
|---|
| Concentrations |
|---|
| Not Available |
|---|
| External Links |
|---|
| DrugBank ID | DB00620 |
|---|
| HMDB ID | HMDB0014758 |
|---|
| FooDB ID | Not Available |
|---|
| Phenol Explorer ID | Not Available |
|---|
| KNApSAcK ID | Not Available |
|---|
| BiGG ID | Not Available |
|---|
| BioCyc ID | Not Available |
|---|
| METLIN ID | Not Available |
|---|
| PDB ID | Not Available |
|---|
| Wikipedia Link | Triamcinolone |
|---|
| Chemspider ID | 29046 |
|---|
| ChEBI ID | 9667 |
|---|
| PubChem Compound ID | 31307 |
|---|
| Kegg Compound ID | Not Available |
|---|
| YMDB ID | Not Available |
|---|
| ECMDB ID | Not Available |
|---|
| References |
|---|
| Synthesis Reference | Abu Alam, “Triamcinolone formulations and methods for their preparation and use.” U.S. Patent US20040186084, issued September 23, 2004. |
|---|
| MSDS | Link |
|---|
| General References | |
|---|