| Record Information |
|---|
| Version | 1.0 |
|---|
| Creation Date | 2014-09-11 02:06:10 UTC |
|---|
| Update Date | 2016-11-09 01:09:11 UTC |
|---|
| Accession Number | CHEM003673 |
|---|
| Identification |
|---|
| Common Name | Teniposide |
|---|
| Class | Small Molecule |
|---|
| Description | A semisynthetic derivative of podophyllotoxin that exhibits antitumor activity. Teniposide inhibits DNA synthesis by forming a complex with topoisomerase II and DNA. This complex induces breaks in double stranded DNA and prevents repair by topoisomerase II binding. Accumulated breaks in DNA prevent cells from entering into the mitotic phase of the cell cycle, and lead to cell death. Teniposide acts primarily in the G2 and S phases of the cycle. |
|---|
| Contaminant Sources | - HMDB Contaminants - Urine
- IARC Carcinogens Group 2A
- STOFF IDENT Compounds
- T3DB toxins
- ToxCast & Tox21 Chemicals
|
|---|
| Contaminant Type | - Antineoplastic Agent
- Drug
- Enzyme Inhibitor
- Ester
- Ether
- Metabolite
- Nucleic Acid Synthesis Inhibitor
- Organic Compound
- Synthetic Compound
|
|---|
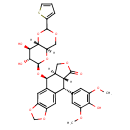
| Chemical Structure | |
|---|
| Synonyms | | Value | Source |
|---|
| 4'-Demethylepipodophyllotoxin 9-(4,6-O-2-thenylidene-beta-D-glucopyranoside) | ChEBI | | 4'-Demethylepipodophyllotoxin-beta-D-thenylidene glucoside | ChEBI | | HSDB 6546 | ChEBI | | NSC 122819 | ChEBI | | NSC-122819 | ChEBI | | Teniposido | ChEBI | | Teniposidum | ChEBI | | VM-26 | ChEBI | | Vumon | ChEBI | | 4'-Demethylepipodophyllotoxin 9-(4,6-O-2-thenylidene-b-D-glucopyranoside) | Generator | | 4'-Demethylepipodophyllotoxin 9-(4,6-O-2-thenylidene-β-D-glucopyranoside) | Generator | | 4'-Demethylepipodophyllotoxin-b-D-thenylidene glucoside | Generator | | 4'-Demethylepipodophyllotoxin-β-D-thenylidene glucoside | Generator | | Bristol myers brand OF teniposide | HMDB | | Bristol myers squibb brand OF teniposide | HMDB | | Bristol-myers brand OF teniposide | HMDB | | Bristol-myers squibb brand OF teniposide | HMDB | | Demethyl epipodophyllotoxin thenylidine glucoside | HMDB | | Teniposide, (5a alpha,9 alpha(s*))-isomer | HMDB | | VM 26 | HMDB |
|
|---|
| Chemical Formula | C32H32O13S |
|---|
| Average Molecular Mass | 656.654 g/mol |
|---|
| Monoisotopic Mass | 656.156 g/mol |
|---|
| CAS Registry Number | 29767-20-2 |
|---|
| IUPAC Name | (10R,11R,15R,16S)-16-{[(4aR,6R,7R,8R,8aS)-7,8-dihydroxy-2-(thiophen-2-yl)-hexahydro-2H-pyrano[3,2-d][1,3]dioxin-6-yl]oxy}-10-(4-hydroxy-3,5-dimethoxyphenyl)-4,6,13-trioxatetracyclo[7.7.0.0³,⁷.0¹¹,¹⁵]hexadeca-1,3(7),8-trien-12-one |
|---|
| Traditional Name | teniposide |
|---|
| SMILES | [H][C@]12COC(=O)[C@]1([H])[C@H](C1=CC(OC)=C(O)C(OC)=C1)C1=CC3=C(OCO3)C=C1[C@H]2O[C@@H]1O[C@]2([H])COC(O[C@@]2([H])[C@H](O)[C@H]1O)C1=CC=CS1 |
|---|
| InChI Identifier | InChI=1S/C32H32O13S/c1-37-19-6-13(7-20(38-2)25(19)33)23-14-8-17-18(42-12-41-17)9-15(14)28(16-10-39-30(36)24(16)23)44-32-27(35)26(34)29-21(43-32)11-40-31(45-29)22-4-3-5-46-22/h3-9,16,21,23-24,26-29,31-35H,10-12H2,1-2H3/t16-,21+,23+,24-,26+,27+,28+,29+,31?,32-/m0/s1 |
|---|
| InChI Key | NRUKOCRGYNPUPR-PSZSYXFXSA-N |
|---|
| Chemical Taxonomy |
|---|
| Description | belongs to the class of inorganic compounds known as metalloid oxides. These are inorganic compounds containing an oxygen atom of an oxidation state of -2, in which the heaviest atom bonded to the oxygen is a metalloid. |
|---|
| Kingdom | Inorganic compounds |
|---|
| Super Class | Mixed metal/non-metal compounds |
|---|
| Class | Metalloid organides |
|---|
| Sub Class | Metalloid oxides |
|---|
| Direct Parent | Metalloid oxides |
|---|
| Alternative Parents | |
|---|
| Substituents | - Metalloid oxide
- Inorganic oxide
- Inorganic salt
- Inorganic metalloid salt
|
|---|
| Molecular Framework | Not Available |
|---|
| External Descriptors | |
|---|
| Biological Properties |
|---|
| Status | Detected and Not Quantified |
|---|
| Origin | Exogenous |
|---|
| Cellular Locations | |
|---|
| Biofluid Locations | Not Available |
|---|
| Tissue Locations | Not Available |
|---|
| Pathways | | Name | SMPDB Link | KEGG Link |
|---|
| Teniposide Pathway | Not Available | Not Available |
|
|---|
| Applications | Not Available |
|---|
| Biological Roles | Not Available |
|---|
| Chemical Roles | Not Available |
|---|
| Physical Properties |
|---|
| State | Solid |
|---|
| Appearance | White powder. |
|---|
| Experimental Properties | | Property | Value |
|---|
| Melting Point | 242-246°C | | Boiling Point | Not Available | | Solubility | 5.98e-02 g/L |
|
|---|
| Predicted Properties | |
|---|
| Spectra |
|---|
| Spectra | | Spectrum Type | Description | Splash Key | View |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | splash10-0a71-5785097000-98bde989946936a415b9 | Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (TMS_1_1) - 70eV, Positive | Not Available | Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (TMS_1_2) - 70eV, Positive | Not Available | Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (TMS_1_3) - 70eV, Positive | Not Available | Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (TMS_2_1) - 70eV, Positive | Not Available | Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (TMS_2_2) - 70eV, Positive | Not Available | Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (TMS_2_3) - 70eV, Positive | Not Available | Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (TMS_3_1) - 70eV, Positive | Not Available | Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (TBDMS_1_1) - 70eV, Positive | Not Available | Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (TBDMS_1_2) - 70eV, Positive | Not Available | Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (TBDMS_1_3) - 70eV, Positive | Not Available | Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (TBDMS_2_1) - 70eV, Positive | Not Available | Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (TBDMS_2_2) - 70eV, Positive | Not Available | Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (TBDMS_2_3) - 70eV, Positive | Not Available | Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS ("Teniposide,1TMS,#1" TMS) - 70eV, Positive | Not Available | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-0zgi-0028609000-2103fbbc9230d944f190 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-0ue9-0119500000-822a8a5e0ce6ead99756 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-0ue9-2219200000-70925247141bc507a296 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-0a5a-0518009000-b551c2cfb3b192aa0c97 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-01ot-1409121000-a632ab719b28e6760ca1 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-0532-3009000000-a2fcbe218ec09c074476 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-0kai-0069518000-4ca4220ba0f0b87931d5 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-001i-0029001000-8525d63245524aa711d7 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-0f80-0129000000-340326fd8e647fd49e25 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-0a4i-0000009000-60dafc658503bb6aca9e | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-0a59-1002019000-4cb4998458f9b7c40d68 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-001i-9103042000-d52ca9fb9e8848a4e5d7 | Spectrum |
|
|---|
| Toxicity Profile |
|---|
| Route of Exposure | Not Available |
|---|
| Mechanism of Toxicity | The mechanism of action appears to be related to the inhibition of type II topoisomerase activity since teniposide does not intercalate into DNA or bind strongly to DNA. Teniposide binds to and inhibits DNA topoisomerase II. The cytotoxic effects of teniposide are related to the relative number of double-stranded DNA breaks produced in cells, which are a reflection of the stabilization of a topoisomerase II-DNA intermediate. |
|---|
| Metabolism | Route of Elimination: From 4% to 12% of a dose is excreted in urine as parent drug. Fecal excretion of radioactivity within 72 hours after dosing accounted for 0% to 10% of the dose.
Half Life: 5 hours |
|---|
| Toxicity Values | Not Available |
|---|
| Lethal Dose | Not Available |
|---|
| Carcinogenicity (IARC Classification) | 2A, probably carcinogenic to humans. (1) |
|---|
| Uses/Sources | Teniposide is used for the treatment of refractory acute lymphoblastic leukaemia |
|---|
| Minimum Risk Level | Not Available |
|---|
| Health Effects | Not Available |
|---|
| Symptoms | Not Available |
|---|
| Treatment | Not Available |
|---|
| Concentrations |
|---|
| Not Available |
|---|
| External Links |
|---|
| DrugBank ID | Not Available |
|---|
| HMDB ID | HMDB0014587 |
|---|
| FooDB ID | Not Available |
|---|
| Phenol Explorer ID | Not Available |
|---|
| KNApSAcK ID | Not Available |
|---|
| BiGG ID | Not Available |
|---|
| BioCyc ID | Not Available |
|---|
| METLIN ID | Not Available |
|---|
| PDB ID | Not Available |
|---|
| Wikipedia Link | Teniposide |
|---|
| Chemspider ID | 31930 |
|---|
| ChEBI ID | 75988 |
|---|
| PubChem Compound ID | 34698 |
|---|
| Kegg Compound ID | C11153 |
|---|
| YMDB ID | Not Available |
|---|
| ECMDB ID | Not Available |
|---|
| References |
|---|
| Synthesis Reference | Not Available |
|---|
| MSDS | Link |
|---|
| General References | |
|---|