| Record Information |
|---|
| Version | 1.0 |
|---|
| Creation Date | 2014-09-11 02:05:24 UTC |
|---|
| Update Date | 2016-11-09 01:09:11 UTC |
|---|
| Accession Number | CHEM003654 |
|---|
| Identification |
|---|
| Common Name | Griseofulvin |
|---|
| Class | Small Molecule |
|---|
| Description | Griseofulvin is only found in individuals that have used or taken this drug. It is an antifungal antibiotic. Griseofulvin may be given by mouth in the treatment of tinea infections. Griseofulvin is fungistatic, however the exact mechanism by which it inhibits the growth of dermatophytes is not clear. It is thought to inhibit fungal cell mitosis and nuclear acid synthesis. It also binds to and interferes with the function of spindle and cytoplasmic microtubules by binding to alpha and beta tubulin. It binds to keratin in human cells, then once it reaches the fungal site of action, it binds to fungal microtubes thus altering the fungal process of mitosis. |
|---|
| Contaminant Sources | - HMDB Contaminants - Urine
- IARC Carcinogens Group 2B
- STOFF IDENT Compounds
- T3DB toxins
- ToxCast & Tox21 Chemicals
|
|---|
| Contaminant Type | - Anti-Bacterial Agent
- Antifungal Agent
- Drug
- Ester
- Ether
- Metabolite
- Organic Compound
- Organochloride
- Synthetic Compound
|
|---|
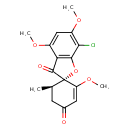
| Chemical Structure | |
|---|
| Synonyms | | Value | Source |
|---|
| (+)-Griseofulvin | ChEBI | | Amudane | ChEBI | | Curling factor | ChEBI | | Fulcin | ChEBI | | Fulvicin | ChEBI | | Grifulvin | ChEBI | | Grisactin | ChEBI | | Griseofulvina | ChEBI | | Griseofulvine | ChEBI | | Griseofulvinum | ChEBI | | Grisovin | ChEBI | | Grysio | ChEBI | | Lamoryl | ChEBI | | Likuden | ChEBI | | Poncyl | ChEBI | | Spirofulvin | ChEBI | | Sporostatin | ChEBI | | GRE | Kegg | | Gris-peg | Kegg | | Grisactin V | Kegg | | Fulvicin u F | HMDB | | Fulvicin-u-F | HMDB | | GrisPEG | HMDB | | Grifulvin V | HMDB | | Gris peg | HMDB | | Grisefuline | HMDB | | V, Grifulvin | HMDB | | FulvicinUF | HMDB |
|
|---|
| Chemical Formula | C17H17ClO6 |
|---|
| Average Molecular Mass | 352.766 g/mol |
|---|
| Monoisotopic Mass | 352.071 g/mol |
|---|
| CAS Registry Number | 126-07-8 |
|---|
| IUPAC Name | (2S,6'R)-7-chloro-2',4,6-trimethoxy-6'-methyl-3H-spiro[1-benzofuran-2,1'-cyclohexan]-2'-ene-3,4'-dione |
|---|
| Traditional Name | griseofulvin |
|---|
| SMILES | COC1=CC(OC)=C(Cl)C2=C1C(=O)[C@]1(O2)[C@H](C)CC(=O)C=C1OC |
|---|
| InChI Identifier | InChI=1S/C17H17ClO6/c1-8-5-9(19)6-12(23-4)17(8)16(20)13-10(21-2)7-11(22-3)14(18)15(13)24-17/h6-8H,5H2,1-4H3/t8-,17+/m1/s1 |
|---|
| InChI Key | DDUHZTYCFQRHIY-RBHXEPJQSA-N |
|---|
| Chemical Taxonomy |
|---|
| Description | belongs to the class of organic compounds known as benzofurans. These are organic compounds containing a benzene ring fused to a furan. Furan is a five-membered aromatic ring with four carbon atoms and one oxygen atom. |
|---|
| Kingdom | Organic compounds |
|---|
| Super Class | Organoheterocyclic compounds |
|---|
| Class | Benzofurans |
|---|
| Sub Class | Not Available |
|---|
| Direct Parent | Benzofurans |
|---|
| Alternative Parents | |
|---|
| Substituents | - Coumaran
- Benzofuran
- Anisole
- Aryl alkyl ketone
- Aryl ketone
- Alkyl aryl ether
- Cyclohexenone
- Aryl chloride
- Aryl halide
- Benzenoid
- Vinylogous ester
- Ketone
- Cyclic ketone
- Ether
- Oxacycle
- Organooxygen compound
- Organochloride
- Organohalogen compound
- Carbonyl group
- Organic oxygen compound
- Hydrocarbon derivative
- Organic oxide
- Aromatic heteropolycyclic compound
|
|---|
| Molecular Framework | Aromatic heteropolycyclic compounds |
|---|
| External Descriptors | |
|---|
| Biological Properties |
|---|
| Status | Detected and Not Quantified |
|---|
| Origin | Exogenous |
|---|
| Cellular Locations | |
|---|
| Biofluid Locations | Not Available |
|---|
| Tissue Locations | Not Available |
|---|
| Pathways | Not Available |
|---|
| Applications | Not Available |
|---|
| Biological Roles | Not Available |
|---|
| Chemical Roles | Not Available |
|---|
| Physical Properties |
|---|
| State | Solid |
|---|
| Appearance | White powder. |
|---|
| Experimental Properties | | Property | Value |
|---|
| Melting Point | 220°C | | Boiling Point | Not Available | | Solubility | 8.64 mg/L (at 25°C) |
|
|---|
| Predicted Properties | |
|---|
| Spectra |
|---|
| Spectra | | Spectrum Type | Description | Splash Key | View |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | splash10-05i1-9208000000-bc69e8498dfe1365cbf9 | Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | Not Available | Spectrum | | LC-MS/MS | LC-MS/MS Spectrum - LC-ESI-qTof , Positive | splash10-0uxr-0589000000-f8705acff560aebbf057 | Spectrum | | LC-MS/MS | LC-MS/MS Spectrum - LC-ESI-qTof , Positive | splash10-0uxr-0469000000-196c982b619369269fc4 | Spectrum | | LC-MS/MS | LC-MS/MS Spectrum - LC-ESI-qTof , Positive | splash10-014i-2960000000-3f14b9f983cc6e713b6a | Spectrum | | LC-MS/MS | LC-MS/MS Spectrum - Linear Ion Trap , positive | splash10-014r-0291000000-6f003312919e202ee091 | Spectrum | | LC-MS/MS | LC-MS/MS Spectrum - , positive | splash10-0uxr-0589000000-f8705acff560aebbf057 | Spectrum | | LC-MS/MS | LC-MS/MS Spectrum - , positive | splash10-0uxr-0469000000-196c982b619369269fc4 | Spectrum | | LC-MS/MS | LC-MS/MS Spectrum - , positive | splash10-014i-2960000000-3f14b9f983cc6e713b6a | Spectrum | | LC-MS/MS | LC-MS/MS Spectrum - 20V, Positive | splash10-014i-0491000000-79844b907995e8371fba | Spectrum | | LC-MS/MS | LC-MS/MS Spectrum - 10V, Positive | splash10-0udi-0239000000-7cd02bae189372507c96 | Spectrum | | LC-MS/MS | LC-MS/MS Spectrum - 40V, Positive | splash10-014i-0940000000-979db7ec6757627784a3 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-0udi-0019000000-6e8e872e3063f2013216 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-0gb9-6359000000-b8b346f6cfebc0766adf | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-052r-7940000000-96dddca714f878c4694c | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-0udi-0009000000-dc216555ed72b685bd2b | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-0udr-0019000000-ff656557d1783726dcf2 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-0a4i-6293000000-1552e154d793f60c7d3e | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-0udi-0009000000-ccd58a14a68a0a179df4 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-0udi-0239000000-8bf9c021181f8ddf16e1 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-0pc0-9351000000-0e6d75fc00dc250731f3 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-0udi-0009000000-da99c723afce93a9bc57 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-0udi-0159000000-e96870a8def0c72a7f50 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-052k-3092000000-e5a2facca3e774b65248 | Spectrum |
|
|---|
| Toxicity Profile |
|---|
| Route of Exposure | Poorly absorbed from GI ranging from 25 to 70% of an oral dose. Absorption is significantly enhanced by administration with or after a fatty meal. |
|---|
| Mechanism of Toxicity | Griseofulvin is fungistatic, however the exact mechanism by which it inhibits the growth of dermatophytes is not clear. It is thought to inhibit fungal cell mitosis and nuclear acid synthesis. It also binds to and interferes with the function of spindle and cytoplasmic microtubules by binding to alpha and beta tubulin. It binds to keratin in human cells, then once it reaches the fungal site of action, it binds to fungal microtubes thus altering the fungal process of mitosis. |
|---|
| Metabolism | Primarily hepatic with major metabolites being 6-methyl-griseofulvin and its glucuronide conjugate.
Half Life: 9-21 hours |
|---|
| Toxicity Values | Not Available |
|---|
| Lethal Dose | Not Available |
|---|
| Carcinogenicity (IARC Classification) | 2B, possibly carcinogenic to humans. (1) |
|---|
| Uses/Sources | Griseofulvin is used both in animals and in humans, to treat fungal infections of the skin (commonly known as ringworm) and nails. Griseofulvin is used orally only for dermatophytosis. It is ineffective topically. Griseofulvin is reserved for cases with nail, hair or large body surface involvement. (Wikipedia) |
|---|
| Minimum Risk Level | Not Available |
|---|
| Health Effects | Not Available |
|---|
| Symptoms | Side effects are minor: headaches, gastrointestinal reactions and cutaneous eruptions |
|---|
| Treatment | Not Available |
|---|
| Concentrations |
|---|
| Not Available |
|---|
| External Links |
|---|
| DrugBank ID | DB00400 |
|---|
| HMDB ID | HMDB0014544 |
|---|
| FooDB ID | Not Available |
|---|
| Phenol Explorer ID | Not Available |
|---|
| KNApSAcK ID | C00002398 |
|---|
| BiGG ID | Not Available |
|---|
| BioCyc ID | CPD-17786 |
|---|
| METLIN ID | Not Available |
|---|
| PDB ID | Not Available |
|---|
| Wikipedia Link | Griseofulvin |
|---|
| Chemspider ID | 389934 |
|---|
| ChEBI ID | 27779 |
|---|
| PubChem Compound ID | 441140 |
|---|
| Kegg Compound ID | C06686 |
|---|
| YMDB ID | Not Available |
|---|
| ECMDB ID | Not Available |
|---|
| References |
|---|
| Synthesis Reference | Gary Liversidge, “Methods of making and using novel griseofulvin compositions.” U.S. Patent US20070098805, issued May 03, 2007. |
|---|
| MSDS | Link |
|---|
| General References | |
|---|