| Record Information |
|---|
| Version | 1.0 |
|---|
| Creation Date | 2014-09-11 02:04:43 UTC |
|---|
| Update Date | 2016-11-09 01:09:11 UTC |
|---|
| Accession Number | CHEM003639 |
|---|
| Identification |
|---|
| Common Name | Carmustine |
|---|
| Class | Small Molecule |
|---|
| Description | Carmustine is a cell-cycle phase nonspecific alkylating antineoplastic agent. It is used in the treatment of brain tumors and various other malignant neoplasms. This substance may reasonably be anticipated to be a carcinogen according to the Fourth Annual Report on Carcinogens (NTP 85-002, 1985). |
|---|
| Contaminant Sources | - FooDB Chemicals
- HMDB Contaminants - Urine
- IARC Carcinogens Group 2A
- STOFF IDENT Compounds
- T3DB toxins
- ToxCast & Tox21 Chemicals
|
|---|
| Contaminant Type | - Amine
- Antineoplastic Agent, Alkylating
- Drug
- Metabolite
- Organic Compound
- Organochloride
- Synthetic Compound
|
|---|
| Chemical Structure | |
|---|
| Synonyms | | Value | Source |
|---|
| BCNU | ChEBI | | Bicnu | ChEBI | | Bischloroethyl nitrosourea | ChEBI | | Carmustina | ChEBI | | Carmustinum | ChEBI | | Gliadel | ChEBI | | N,N'-bis(2-chloroethyl)-N-nitrosourea | ChEBI | | Bischlorethylnitrosourea | HMDB | | Bischlorethylnitrosurea | HMDB | | Carmustin | HMDB | | FIVB | HMDB | | Nitrumon | HMDB | | 1,3-Bis(2-chloroethyl)-1-nitrosourea | HMDB |
|
|---|
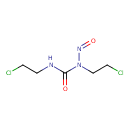
| Chemical Formula | C5H9Cl2N3O2 |
|---|
| Average Molecular Mass | 214.050 g/mol |
|---|
| Monoisotopic Mass | 213.007 g/mol |
|---|
| CAS Registry Number | 154-93-8 |
|---|
| IUPAC Name | 1,3-bis(2-chloroethyl)-3-nitrosourea |
|---|
| Traditional Name | carmustine |
|---|
| SMILES | ClCCNC(=O)N(CCCl)N=O |
|---|
| InChI Identifier | InChI=1S/C5H9Cl2N3O2/c6-1-3-8-5(11)10(9-12)4-2-7/h1-4H2,(H,8,11) |
|---|
| InChI Key | DLGOEMSEDOSKAD-UHFFFAOYSA-N |
|---|
| Chemical Taxonomy |
|---|
| Description | belongs to the class of organic compounds known as nitrosoureas. Nitrosoureas are compounds containing a nitro group and an urea group N-N linked together, with the general structure R1N(R2)C(=O)N(R3)N=O. |
|---|
| Kingdom | Organic compounds |
|---|
| Super Class | Organic acids and derivatives |
|---|
| Class | Organic carbonic acids and derivatives |
|---|
| Sub Class | Ureas |
|---|
| Direct Parent | Nitrosoureas |
|---|
| Alternative Parents | |
|---|
| Substituents | - Nitrosourea
- Semicarbazide
- Nitrosamide
- Organic n-nitroso compound
- Organic nitroso compound
- Organic oxygen compound
- Organopnictogen compound
- Organic oxide
- Hydrocarbon derivative
- Alkyl chloride
- Organooxygen compound
- Organonitrogen compound
- Organochloride
- Organohalogen compound
- Carbonyl group
- Alkyl halide
- Organic nitrogen compound
- Aliphatic acyclic compound
|
|---|
| Molecular Framework | Aliphatic acyclic compounds |
|---|
| External Descriptors | |
|---|
| Biological Properties |
|---|
| Status | Detected and Not Quantified |
|---|
| Origin | Exogenous |
|---|
| Cellular Locations | - Cytoplasm
- Extracellular
- Membrane
|
|---|
| Biofluid Locations | Not Available |
|---|
| Tissue Locations | Not Available |
|---|
| Pathways | Not Available |
|---|
| Applications | Not Available |
|---|
| Biological Roles | Not Available |
|---|
| Chemical Roles | Not Available |
|---|
| Physical Properties |
|---|
| State | Solid |
|---|
| Appearance | White powder. |
|---|
| Experimental Properties | | Property | Value |
|---|
| Melting Point | 31°C | | Boiling Point | Not Available | | Solubility | 4000 mg/L (at 25°C) |
|
|---|
| Predicted Properties | |
|---|
| Spectra |
|---|
| Spectra | | Spectrum Type | Description | Splash Key | View |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | splash10-0bu0-6900000000-7ee333c26479b9275253 | Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | Not Available | Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | Not Available | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-06vi-9630000000-1b262631b4a720cd9eba | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-0a6r-9500000000-fbebb9877cfb22d2c3d8 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-03fr-9000000000-86de321ebee3984d7705 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-116u-4930000000-a2c48761c56e3640e06b | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-004l-6900000000-fc62a96dc02889b5a3b7 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-004l-9200000000-d116bdf5580083ca15c7 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-03di-4390000000-26b54291487efd0acb6f | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-03di-9400000000-52b9e7457ec89c9907c2 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-03di-9000000000-d239cf72a61995f936f3 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-03di-4390000000-a11de64a3d45d4733ab2 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-001i-9200000000-a9b03f4595d1bb5cb7d2 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-001i-9200000000-14857476fd37bc517cbb | Spectrum |
|
|---|
| Toxicity Profile |
|---|
| Route of Exposure | Not Available |
|---|
| Mechanism of Toxicity | Carmustine causes cross-links in DNA and RNA, leading to the inhibition of DNA synthesis, RNA production and RNA translation (protein synthesis). Carmustine also binds to and modifies (carbamoylates) glutathione reductase. This leads to cell death. |
|---|
| Metabolism | Hepatic and rapid with active metabolites. Metabolites may persist in the plasma for several days.
Route of Elimination: Approximately 60% to 70% of a total dose is excreted in the urine in 96 hours and about 10% as respiratory CO2.
Half Life: 15-30 minutes |
|---|
| Toxicity Values | The oral LD50s in rat and mouse are 20 mg/kg and 45 mg/kg, respectively. |
|---|
| Lethal Dose | Not Available |
|---|
| Carcinogenicity (IARC Classification) | 2A, probably carcinogenic to humans. (1) |
|---|
| Uses/Sources | For the treatment of brain tumors, multiple myeloma, Hodgkin's disease and Non-Hodgkin's lymphomas. |
|---|
| Minimum Risk Level | Not Available |
|---|
| Health Effects | Not Available |
|---|
| Symptoms | Not Available |
|---|
| Treatment | Not Available |
|---|
| Concentrations |
|---|
| Not Available |
|---|
| External Links |
|---|
| DrugBank ID | DB00262 |
|---|
| HMDB ID | HMDB0014407 |
|---|
| FooDB ID | FDB012974 |
|---|
| Phenol Explorer ID | Not Available |
|---|
| KNApSAcK ID | Not Available |
|---|
| BiGG ID | Not Available |
|---|
| BioCyc ID | Not Available |
|---|
| METLIN ID | Not Available |
|---|
| PDB ID | Not Available |
|---|
| Wikipedia Link | Carmustine |
|---|
| Chemspider ID | 2480 |
|---|
| ChEBI ID | 3423 |
|---|
| PubChem Compound ID | 2578 |
|---|
| Kegg Compound ID | C06873 |
|---|
| YMDB ID | Not Available |
|---|
| ECMDB ID | Not Available |
|---|
| References |
|---|
| Synthesis Reference | Not Available |
|---|
| MSDS | Link |
|---|
| General References | Not Available |
|---|