| Record Information |
|---|
| Version | 1.0 |
|---|
| Creation Date | 2014-08-30 21:06:06 UTC |
|---|
| Update Date | 2016-11-09 01:09:09 UTC |
|---|
| Accession Number | CHEM003531 |
|---|
| Identification |
|---|
| Common Name | Ketazolam |
|---|
| Class | Small Molecule |
|---|
| Description | Ketazolam is a drug which is a benzodiazepine derivative. It possesses anxiolytic, anticonvulsant, sedative and skeletal muscle relaxant properties. Ketazolam is not approved for sale in the United States or Canada. |
|---|
| Contaminant Sources | - HMDB Contaminants - Urine
- STOFF IDENT Compounds
- T3DB toxins
|
|---|
| Contaminant Type | - Amide
- Amine
- Benzodiazepine
- Drug
- Ether
- Organic Compound
- Organochloride
- Synthetic Compound
|
|---|
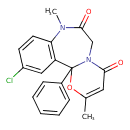
| Chemical Structure | |
|---|
| Synonyms | | Value | Source |
|---|
| Marcen | HMDB | | Sedotime | HMDB | | Solatran | HMDB | | Unakalm | HMDB |
|
|---|
| Chemical Formula | C20H17ClN2O3 |
|---|
| Average Molecular Mass | 368.814 g/mol |
|---|
| Monoisotopic Mass | 368.093 g/mol |
|---|
| CAS Registry Number | 27223-35-4 |
|---|
| IUPAC Name | 14-chloro-4,10-dimethyl-2-phenyl-3-oxa-7,10-diazatricyclo[9.4.0.0²,⁷]pentadeca-1(11),4,12,14-tetraene-6,9-dione |
|---|
| Traditional Name | ketazolam |
|---|
| SMILES | CN1C2=C(C=C(Cl)C=C2)C2(OC(C)=CC(=O)N2CC1=O)C1=CC=CC=C1 |
|---|
| InChI Identifier | InChI=1S/C20H17ClN2O3/c1-13-10-18(24)23-12-19(25)22(2)17-9-8-15(21)11-16(17)20(23,26-13)14-6-4-3-5-7-14/h3-11H,12H2,1-2H3 |
|---|
| InChI Key | PWAJCNITSBZRBL-UHFFFAOYSA-N |
|---|
| Chemical Taxonomy |
|---|
| Description | belongs to the class of organic compounds known as 1,4-benzodiazepines. These are organic compounds containing a benzene ring fused to a 1,4-azepine. |
|---|
| Kingdom | Organic compounds |
|---|
| Super Class | Organoheterocyclic compounds |
|---|
| Class | Benzodiazepines |
|---|
| Sub Class | 1,4-benzodiazepines |
|---|
| Direct Parent | 1,4-benzodiazepines |
|---|
| Alternative Parents | |
|---|
| Substituents | - 1,4-benzodiazepine
- Alpha-amino acid or derivatives
- Aryl chloride
- Aryl halide
- Monocyclic benzene moiety
- Benzenoid
- Tertiary carboxylic acid amide
- Vinylogous ester
- Carboxamide group
- Lactam
- Carboxylic acid derivative
- Oxacycle
- Azacycle
- Organochloride
- Organohalogen compound
- Organic nitrogen compound
- Hydrocarbon derivative
- Carbonyl group
- Organic oxide
- Organopnictogen compound
- Organonitrogen compound
- Organooxygen compound
- Organic oxygen compound
- Aromatic heteropolycyclic compound
|
|---|
| Molecular Framework | Aromatic heteropolycyclic compounds |
|---|
| External Descriptors | Not Available |
|---|
| Biological Properties |
|---|
| Status | Detected and Not Quantified |
|---|
| Origin | Exogenous |
|---|
| Cellular Locations | |
|---|
| Biofluid Locations | Not Available |
|---|
| Tissue Locations | Not Available |
|---|
| Pathways | Not Available |
|---|
| Applications | Not Available |
|---|
| Biological Roles | Not Available |
|---|
| Chemical Roles | Not Available |
|---|
| Physical Properties |
|---|
| State | Solid |
|---|
| Appearance | White powder. |
|---|
| Experimental Properties | | Property | Value |
|---|
| Melting Point | 174-176°C | | Boiling Point | Not Available | | Solubility | Not Available |
|
|---|
| Predicted Properties | |
|---|
| Spectra |
|---|
| Spectra | | Spectrum Type | Description | Splash Key | View |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | splash10-0fri-7794000000-e5f97e7007c42a0e6cae | Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | Not Available | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-014i-0009000000-00a0ebc61e31ee5f9fb7 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-014i-1009000000-af371ac384c19582e8cc | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-00kf-7910000000-9de91642147606a3f899 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-014i-0009000000-452e71c58ce51c808f39 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-0002-0094000000-854abc1e120fa24f40b8 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-053v-7293000000-24656d4d8f4834731919 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-014i-0009000000-940bacac6459ea82189e | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-014i-0019000000-15e1445eaba0a4c42fa1 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-0uy4-0295000000-0beb62e6599fbcc102b1 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-014i-0009000000-de71073cb0d517e7b61e | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-014i-0009000000-de71073cb0d517e7b61e | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-014i-5029000000-c65a5a034b12b7c917a6 | Spectrum | | MS | Mass Spectrum (Electron Ionization) | splash10-0a59-1290000000-7ef8a75bf466c745a728 | Spectrum |
|
|---|
| Toxicity Profile |
|---|
| Route of Exposure | Not Available |
|---|
| Mechanism of Toxicity | Benzodiazepines share a similar chemical structure and their effects in humans are mainly produced by the allosteric modification of a specific kind of neurotransmitter receptor, the GABAA receptor, which increases the conductance of this inhibitory channel; this results in the various therapeutic effects as well as adverse effects of benzodiazepines. Binding of benzodiazepines to this receptor complex promotes binding of GABA, which in turn increases the conduction of chloride ions across the neuronal cell membrane. This increased conductance raises the membrane potential of the neuron resulting in inhibition of neuronal firing. In addition, different GABAA receptor subtypes have varying distributions within different regions of the brain and therefore control distinct neuronal circuits. Hence, activation of different GABAA receptor subtypes by benzodiazepines may result in distinct pharmacological actions. |
|---|
| Metabolism | Ketazolam breaks down in the blood to diazepam which breaks down to demoxepam which breaks down to desmethyldiazepam.
Route of Elimination: Diazepam and its metabolites are excreted mainly in the urine, predominantly as their glucuronide conjugates.
Half Life: 26-200 hours |
|---|
| Toxicity Values | Not Available |
|---|
| Lethal Dose | Not Available |
|---|
| Carcinogenicity (IARC Classification) | No indication of carcinogenicity to humans (not listed by IARC). |
|---|
| Uses/Sources | Ketazolam could be used for the treatment of anxiety. In approved countries, it is indicated for the treatment of anxiety, tension, irritability and similar stress related symptoms. |
|---|
| Minimum Risk Level | Not Available |
|---|
| Health Effects | Not Available |
|---|
| Symptoms | Symptoms of overdose include somnolence, confusion, coma, and diminished reflexes. Respiration, pulse and blood pressure should be monitored. |
|---|
| Treatment | General supportive measures should be employed, along with intravenous fluids, and an adequate airway maintained. Hypotension may be combated by the use of norepinephrine or metaraminol. Dialysis is of limited value. Flumazenil (Anexate) is a competitive benzodiazepine receptor antagonist that can be used as an antidote for benzodiazepine overdose. In particular, flumazenil is very effective at reversing the CNS depression associated with benzodiazepines but is less effective at reversing respiratory depression. Its use, however, is controversial as it has numerous contraindications. It is contraindicated in patients who are on long-term benzodiazepines, those who have ingested a substance that lowers the seizure threshold, or in patients who have tachycardia or a history of seizures. As a general rule, medical observation and supportive care are the mainstay of treatment of benzodiazepine overdose. Although benzodiazepines are absorbed by activated charcoal, gastric decontamination with activated charcoal is not beneficial in pure benzodiazepine overdose as the risk of adverse effects often outweigh any potential benefit from the procedure. It is recommended only if benzodiazepines have been taken in combination with other drugs that may benefit from decontamination. Gastric lavage (stomach pumping) or whole bowel irrigation are also not recommended. |
|---|
| Concentrations |
|---|
| Not Available |
|---|
| External Links |
|---|
| DrugBank ID | DB01587 |
|---|
| HMDB ID | HMDB0015526 |
|---|
| FooDB ID | Not Available |
|---|
| Phenol Explorer ID | Not Available |
|---|
| KNApSAcK ID | Not Available |
|---|
| BiGG ID | Not Available |
|---|
| BioCyc ID | Not Available |
|---|
| METLIN ID | Not Available |
|---|
| PDB ID | Not Available |
|---|
| Wikipedia Link | Ketazolam |
|---|
| Chemspider ID | 31110 |
|---|
| ChEBI ID | Not Available |
|---|
| PubChem Compound ID | 33746 |
|---|
| Kegg Compound ID | Not Available |
|---|
| YMDB ID | Not Available |
|---|
| ECMDB ID | Not Available |
|---|
| References |
|---|
| Synthesis Reference | U.S. Patent 3,575,965. |
|---|
| MSDS | Not Available |
|---|
| General References | Not Available |
|---|