| Record Information |
|---|
| Version | 1.0 |
|---|
| Creation Date | 2014-08-30 21:04:54 UTC |
|---|
| Update Date | 2016-11-09 01:09:09 UTC |
|---|
| Accession Number | CHEM003523 |
|---|
| Identification |
|---|
| Common Name | Dexmethylphenidate |
|---|
| Class | Small Molecule |
|---|
| Description | Dexmethylphenidate is the dextrorotary form of methylphenidate. It is a norepinephrine-dopamine reuptake inhibitor (NDRI) and thus a psychostimulant. It is used for treatment of Attention Deficit Hyperactivity Disorder (ADHD). |
|---|
| Contaminant Sources | - HMDB Contaminants - Urine
- T3DB toxins
|
|---|
| Contaminant Type | - Amine
- Drug
- Ester
- Ether
- Organic Compound
- Synthetic Compound
|
|---|
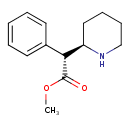
| Chemical Structure | |
|---|
| Synonyms | | Value | Source |
|---|
| (+)-Threo-methylphenidate | ChEBI | | D-Threo-methylphenidate | ChEBI | | Dexmethylphenidatum | ChEBI | | Dexmetilfenidato | ChEBI | | Methyl (R)-phenyl[(R)-piperidin-2-yl]acetate | ChEBI | | (+)-Threo-methylphenidic acid | Generator | | D-Threo-methylphenidic acid | Generator | | Methyl (R)-phenyl[(R)-piperidin-2-yl]acetic acid | Generator | | Dexmethylphenidic acid | Generator | | Dexmethylphenidate hydrochloride | HMDB | | Focalin XR | HMDB | | Hydrochloride, dexmethylphenidate | HMDB | | XR, Focalin | HMDB | | Focalin | HMDB |
|
|---|
| Chemical Formula | C14H19NO2 |
|---|
| Average Molecular Mass | 233.306 g/mol |
|---|
| Monoisotopic Mass | 233.142 g/mol |
|---|
| CAS Registry Number | 40431-64-9 |
|---|
| IUPAC Name | methyl (2R)-2-phenyl-2-[(2R)-piperidin-2-yl]acetate |
|---|
| Traditional Name | dexmethylphenidate |
|---|
| SMILES | COC(=O)[C@@H]([C@H]1CCCCN1)C1=CC=CC=C1 |
|---|
| InChI Identifier | InChI=1S/C14H19NO2/c1-17-14(16)13(11-7-3-2-4-8-11)12-9-5-6-10-15-12/h2-4,7-8,12-13,15H,5-6,9-10H2,1H3/t12-,13-/m1/s1 |
|---|
| InChI Key | DUGOZIWVEXMGBE-CHWSQXEVSA-N |
|---|
| Chemical Taxonomy |
|---|
| Description | belongs to the class of organic compounds known as aralkylamines. These are alkylamines in which the alkyl group is substituted at one carbon atom by an aromatic hydrocarbyl group. |
|---|
| Kingdom | Organic compounds |
|---|
| Super Class | Organic nitrogen compounds |
|---|
| Class | Organonitrogen compounds |
|---|
| Sub Class | Amines |
|---|
| Direct Parent | Aralkylamines |
|---|
| Alternative Parents | |
|---|
| Substituents | - Aralkylamine
- Monocyclic benzene moiety
- Piperidine
- Benzenoid
- Methyl ester
- Amino acid or derivatives
- Carboxylic acid ester
- Carboxylic acid derivative
- Secondary aliphatic amine
- Monocarboxylic acid or derivatives
- Secondary amine
- Azacycle
- Organoheterocyclic compound
- Organopnictogen compound
- Organooxygen compound
- Hydrocarbon derivative
- Carbonyl group
- Organic oxygen compound
- Organic oxide
- Aromatic heteromonocyclic compound
|
|---|
| Molecular Framework | Aromatic heteromonocyclic compounds |
|---|
| External Descriptors | |
|---|
| Biological Properties |
|---|
| Status | Detected and Not Quantified |
|---|
| Origin | Exogenous |
|---|
| Cellular Locations | |
|---|
| Biofluid Locations | Not Available |
|---|
| Tissue Locations | Not Available |
|---|
| Pathways | Not Available |
|---|
| Applications | Not Available |
|---|
| Biological Roles | Not Available |
|---|
| Chemical Roles | Not Available |
|---|
| Physical Properties |
|---|
| State | Solid |
|---|
| Appearance | White powder. |
|---|
| Experimental Properties | | Property | Value |
|---|
| Melting Point | Not Available | | Boiling Point | Not Available | | Solubility | Not Available |
|
|---|
| Predicted Properties | |
|---|
| Spectra |
|---|
| Spectra | | Spectrum Type | Description | Splash Key | View |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | splash10-0089-9610000000-8e4c1241e36274356032 | Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | Not Available | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-001i-1190000000-8803b6fb1d54baf20a6f | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-001i-6390000000-63f6ddbd32540d6c0e4e | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-001i-9500000000-dab6b5667313005cbcd2 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-001i-0090000000-dbf1d73eb0c9410efbf6 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-001i-2290000000-4a7ee5089439605a9759 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-00w9-9520000000-aa484f1df63a11905d5a | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-001i-2190000000-aa6695fd8b35db2e4b41 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-001i-9440000000-3e2430ca32eb71f561e4 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-001i-9300000000-2d49d7918e562aad8156 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-001i-0090000000-96a8c87419b47af6da2a | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-00lr-4790000000-8bbed2d476f383bd9813 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-0006-9400000000-dabf09c5d3b6f3c788fe | Spectrum |
|
|---|
| Toxicity Profile |
|---|
| Route of Exposure | 11-52% |
|---|
| Mechanism of Toxicity | Methylphenidate blocks dopamine uptake in central adrenergic neurons by blocking dopamine transport or carrier proteins. Methylphenidate acts at the brain stem arousal system and the cerebral cortex and causes increased sympathomimetic activity in the central nervous system.
Methylphenidate is a catecholamine reuptake inhibitor that indirectly increases catecholaminergic neurotransmission by inhibiting the dopamine transporter (DAT) and norepinephrine transporter (NET), which are responsible for clearing catecholamines from the synapse, particularly in the striatum and meso-limbic system. |
|---|
| Metabolism | epatic, methylphenidate is metabolized primarily by de-esterification to ritalinic acid (α-phenyl-2-piperidine acetic acid, PPAA), which has little to no pharmacologic activity.
Route of Elimination: Renal
Half Life: 2-4 hours |
|---|
| Toxicity Values | Oral, Mouse: LD50=190mg/kg |
|---|
| Lethal Dose | Not Available |
|---|
| Carcinogenicity (IARC Classification) | No indication of carcinogenicity to humans (not listed by IARC). |
|---|
| Uses/Sources | Dexmethylphenidate is used as a treatment for ADHD, ideally in conjunction with psychological, educational, behavioral or other forms of treatment. |
|---|
| Minimum Risk Level | Not Available |
|---|
| Health Effects | Not Available |
|---|
| Symptoms | Symptoms of overdose include vomiting, agitation, tremors, hyperreflexia, muscle twitching, convulsions (may be followed by coma), euphoria, confusion, hallucinations, delirium, sweating, flushing, headache, hyperpyrexia, tachycardia, palpitations, cardiac arrhythmias, hypertension, mydriasis, and dryness of mucous membranes. |
|---|
| Treatment | Not Available |
|---|
| Concentrations |
|---|
| Not Available |
|---|
| External Links |
|---|
| DrugBank ID | DB06701 |
|---|
| HMDB ID | HMDB0015647 |
|---|
| FooDB ID | Not Available |
|---|
| Phenol Explorer ID | Not Available |
|---|
| KNApSAcK ID | Not Available |
|---|
| BiGG ID | Not Available |
|---|
| BioCyc ID | Not Available |
|---|
| METLIN ID | Not Available |
|---|
| PDB ID | Not Available |
|---|
| Wikipedia Link | Dexmethylphenidate |
|---|
| Chemspider ID | 135807 |
|---|
| ChEBI ID | 51860 |
|---|
| PubChem Compound ID | 154101 |
|---|
| Kegg Compound ID | Not Available |
|---|
| YMDB ID | Not Available |
|---|
| ECMDB ID | Not Available |
|---|
| References |
|---|
| Synthesis Reference | Arie Gutman, “Process for the preparation of dexmethylphenidate hydrochloride.” U.S. Patent US20040180928, issued September 16, 2004. |
|---|
| MSDS | Not Available |
|---|
| General References | Not Available |
|---|