| Record Information |
|---|
| Version | 1.0 |
|---|
| Creation Date | 2014-08-30 21:03:50 UTC |
|---|
| Update Date | 2016-10-28 10:00:48 UTC |
|---|
| Accession Number | CHEM003515 |
|---|
| Identification |
|---|
| Common Name | Pseudoephedrine |
|---|
| Class | Small Molecule |
|---|
| Description | An alpha- and beta-adrenergic agonist that may also enhance release of norepinephrine. It has been used in the treatment of several disorders including asthma, heart failure, rhinitis, and urinary incontinence, and for its central nervous system stimulatory effects in the treatment of narcolepsy and depression. It has become less extensively used with the advent of more selective agonists. [PubChem] |
|---|
| Contaminant Sources | - DEA Chemicals
- FooDB Chemicals
- STOFF IDENT Compounds
- T3DB toxins
|
|---|
| Contaminant Type | - Amine
- Drug
- Food Toxin
- Organic Compound
- Synthetic Compound
|
|---|
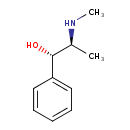
| Chemical Structure | |
|---|
| Synonyms | | Value | Source |
|---|
| (+) Threo-2-(methylamino)-1-phenyl-1-propanol | ChEBI | | (+)-(1S,2S)-Pseudoephedrine | ChEBI | | (+)-Pseudoephedrine | ChEBI | | (+)-Psi-ephedrine | ChEBI | | (+)-Threo-ephedrine | ChEBI | | (1S,2S)-(+)-Pseudoephedrine | ChEBI | | (1S,2S)-Pseudoephedrine | ChEBI | | 2-(Methylamino)-1-phenyl-1-propanol | ChEBI | | D-Isoephedrine | ChEBI | | D-Pseudoephedrine | ChEBI | | D-Psi-2-methylamino-1-phenyl-1-propanol | ChEBI | | D-Psi-ephedrine | ChEBI | | Isoephedrine | ChEBI | | L(+)-Psi-ephedrine | ChEBI | | L-(+)-Pseudoephedrine | ChEBI | | Pseudoefedrina | ChEBI | | Pseudoephedrine D-form | ChEBI | | Pseudoephedrinum | ChEBI | | Psi-ephedrin | ChEBI | | Psi-ephedrine | ChEBI | | trans-Ephedrine | ChEBI | | Neodurasina | Kegg | | Acunaso | Kegg | | 1-Ephedrine | HMDB | | Besan | HMDB | | D-Pseudoephedrine base | HMDB | | Novafed | HMDB | | Pseudoephedrine ephedrine | HMDB | | Sudafed | HMDB | | Ephedrine threo isomer | HMDB | | Pseudoephedrine HCL | HMDB | | Threo isomer OF ephedrine | HMDB | | Pseudoephedrine hydrochloride | HMDB |
|
|---|
| Chemical Formula | C10H15NO |
|---|
| Average Molecular Mass | 165.232 g/mol |
|---|
| Monoisotopic Mass | 165.115 g/mol |
|---|
| CAS Registry Number | 90-82-4 |
|---|
| IUPAC Name | (1S,2S)-2-(methylamino)-1-phenylpropan-1-ol |
|---|
| Traditional Name | pseudoephedrine |
|---|
| SMILES | CN[C@@H](C)[C@@H](O)C1=CC=CC=C1 |
|---|
| InChI Identifier | InChI=1S/C10H15NO/c1-8(11-2)10(12)9-6-4-3-5-7-9/h3-8,10-12H,1-2H3/t8-,10+/m0/s1 |
|---|
| InChI Key | KWGRBVOPPLSCSI-WCBMZHEXSA-N |
|---|
| Chemical Taxonomy |
|---|
| Description | belongs to the class of organic compounds known as phenylpropanes. These are organic compounds containing a phenylpropane moiety. |
|---|
| Kingdom | Organic compounds |
|---|
| Super Class | Benzenoids |
|---|
| Class | Benzene and substituted derivatives |
|---|
| Sub Class | Phenylpropanes |
|---|
| Direct Parent | Phenylpropanes |
|---|
| Alternative Parents | |
|---|
| Substituents | - Phenylpropane
- Aralkylamine
- 1,2-aminoalcohol
- Secondary alcohol
- Secondary aliphatic amine
- Secondary amine
- Alcohol
- Organopnictogen compound
- Organic oxygen compound
- Organooxygen compound
- Organonitrogen compound
- Organic nitrogen compound
- Amine
- Aromatic alcohol
- Hydrocarbon derivative
- Aromatic homomonocyclic compound
|
|---|
| Molecular Framework | Aromatic homomonocyclic compounds |
|---|
| External Descriptors | |
|---|
| Biological Properties |
|---|
| Status | Detected and Not Quantified |
|---|
| Origin | Exogenous |
|---|
| Cellular Locations | |
|---|
| Biofluid Locations | Not Available |
|---|
| Tissue Locations | Not Available |
|---|
| Pathways | Not Available |
|---|
| Applications | |
|---|
| Biological Roles | |
|---|
| Chemical Roles | Not Available |
|---|
| Physical Properties |
|---|
| State | Solid |
|---|
| Appearance | White powder. |
|---|
| Experimental Properties | | Property | Value |
|---|
| Melting Point | 119 °C | | Boiling Point | Not Available | | Solubility | 7 mg/L |
|
|---|
| Predicted Properties | |
|---|
| Spectra |
|---|
| Spectra | | Spectrum Type | Description | Splash Key | View |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | splash10-0a4i-9200000000-0c3fe6959703a851b9cc | Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (1 TMS) - 70eV, Positive | splash10-05cr-4900000000-d9ac11e33b32a9e427ad | Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | Not Available | Spectrum | | LC-MS/MS | LC-MS/MS Spectrum - Quattro_QQQ 10V, N/A (Annotated) | splash10-016s-3900000000-868bf4bba93d8b4be696 | Spectrum | | LC-MS/MS | LC-MS/MS Spectrum - Quattro_QQQ 25V, N/A (Annotated) | splash10-0159-2900000000-6c55fc67b7576cb42a8a | Spectrum | | LC-MS/MS | LC-MS/MS Spectrum - Quattro_QQQ 40V, N/A (Annotated) | splash10-0006-9700000000-459686cbaf7d3b59c202 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-00kb-0900000000-677d622716b4c2545f16 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-00kb-2900000000-416def0e527ddf116a39 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-0aou-9500000000-155625a572f19610e138 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-03di-0900000000-ea7e1b550b5dc0d6d41d | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-03dj-2900000000-f4de25860574bb60daf1 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-0a6r-9600000000-62e375458e5f83795e90 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-0297-3900000000-4496fbcd8548f7ccc58a | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-014i-1900000000-03e637debccd8d202e3c | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-004i-9100000000-7ead088c2f003219a1cf | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-0002-0900000000-0282f3e8fc4bf9296a38 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-014m-3900000000-8ee8987e88c6a5601ef7 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-0006-9300000000-e3932e53d7936649b8db | Spectrum | | MS | Mass Spectrum (Electron Ionization) | splash10-0a4i-9000000000-8f0484f72b48017f1f2e | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 2D NMR | [1H,13C] 2D NMR Spectrum | Not Available | Spectrum |
|
|---|
| Toxicity Profile |
|---|
| Route of Exposure | Pseudoephedrine is readily and almost completely absorbed from the GI tract and there is no evidence of first-pass metabolism. |
|---|
| Mechanism of Toxicity | Pseudoephedrine acts directly on both alpha- and, to a lesser degree, beta-adrenergic receptors. Through direct action on alpha-adrenergic receptors in the mucosa of the respiratory tract, pseudoephedrine produces vasoconstriction. Pseudoephedrine relaxes bronchial smooth muscle by stimulating beta2-adrenergic receptors. Like ephedrine, pseudoephedrine releasing norepinephrine from its storage sites, an indirect effect. This is its main and direct mechanism of action. The displaced noradrenaline is released into the neuronal synapse where it is free to activate the postsynaptic adrenergic receptors. |
|---|
| Metabolism | Hepatic.
Half Life: 9-16 hours |
|---|
| Toxicity Values | Not Available |
|---|
| Lethal Dose | Not Available |
|---|
| Carcinogenicity (IARC Classification) | No indication of carcinogenicity to humans (not listed by IARC). |
|---|
| Uses/Sources | For the treatment of nasal congestion, sinus congestion, Eustachian tube congestion, and vasomotor rhinitis, and as an adjunct to other agents in the optimum treatment of allergic rhinitis, croup, sinusitis, otitis media, and tracheobronchitis. Also used as first-line therapy of priapism. |
|---|
| Minimum Risk Level | Not Available |
|---|
| Health Effects | Not Available |
|---|
| Symptoms | Common adverse reactions include nervousness, restlessness, and insomnia. Rare adverse reactions include difficult/painful urination, dizziness/lightheadedness, heart palpitations, headache, increased sweating, nausea/vomiting, trembling, troubled breathing, unusual paleness, and weakness. |
|---|
| Treatment | Not Available |
|---|
| Concentrations |
|---|
| Not Available |
|---|
| External Links |
|---|
| DrugBank ID | DB00852 |
|---|
| HMDB ID | HMDB0001943 |
|---|
| FooDB ID | FDB022758 |
|---|
| Phenol Explorer ID | Not Available |
|---|
| KNApSAcK ID | C00031097 |
|---|
| BiGG ID | Not Available |
|---|
| BioCyc ID | CPD-9954 |
|---|
| METLIN ID | 2189 |
|---|
| PDB ID | Not Available |
|---|
| Wikipedia Link | Pseudoephedrine |
|---|
| Chemspider ID | 6761 |
|---|
| ChEBI ID | 51209 |
|---|
| PubChem Compound ID | 7028 |
|---|
| Kegg Compound ID | C02765 |
|---|
| YMDB ID | Not Available |
|---|
| ECMDB ID | Not Available |
|---|
| References |
|---|
| Synthesis Reference | Andrew G. Myers, “Synthesis of l-azatyrosine using pseudoephedrine as a chiral auxiliary.” U.S. Patent US5760237, issued June, 1991. |
|---|
| MSDS | Not Available |
|---|
| General References | |
|---|